For our latest grant programs please see Escher Fund for Autism website.
_____________________________________________________________________________________________________________________
ARCHIVES OF PAST GRANT PROGRAMS
RFP: $30,000 grants available; Expressions of interest due March 31, 2023
2023 Grant Program: Genetic, Epigenetic and/or Cellular Impacts of Germ Cell Exposure to Toxicants
ARCHIVES OF PAST GRANT PROGRAMS
RFP: $30,000 grants available; Expressions of interest due March 31, 2023
2023 Grant Program: Genetic, Epigenetic and/or Cellular Impacts of Germ Cell Exposure to Toxicants
• Studies may be in male and/or female germ cells; in mammalian models and/or human cohorts
• Investigation of heritable impacts preferred but not required
• Deadline: March 31, 2023
Background
Autism is a strongly heritable disorder that has, paradoxically, proven to be only weakly genetic, with rare genetic errors accounting for about 10-14% of cases. While many in the field now presume the "missing heritability" of autism can be found in common variants, The Escher Fund for Autism takes a different approach. We are concerned that much of the missing heritability of autism, along with much of its sharply increasing prevalence, is more likely to be found in the under-researched realm of germ cell toxicology.
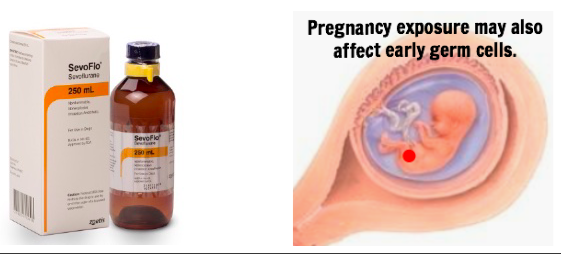
In the modern world, human germ cells, egg and sperm and their precursors, do not arise in a pristine vacuum of biological perfection. Over the course of their development, our germ cells can suffer any number of toxicological challenges, which in some cases may reshape their molecular programming and ultimately their blueprint for gene expression in the offspring. While the vast majority of exposures likely have little to no effect, certain exposures in certain windows of vulnerability may cause pathology in the next generation, as demonstrated widely in mammalian models. Some of these exposures are discussed in Escher et al. 2022 (halogenated inhalational general anesthesia such as sevoflurane, smoking, synthetic steroids, HDAC inhibitors), with another example seen in Kaplanis et al. 2022 (chemotherapy drugs which lead to hypermutation in sperm).
Due to large gaps in knowledge in this field, this round of grants aims to encourage pilot studies investigating the impact of potent toxicants on male and/or female germ cells in mammalian models and/or human cohorts. While it is always preferable to investigate heritable impacts of germline exposures, the only required endpoints for this particular round of grants are the impacts on the germ cells (male and/or female) themselves.
Researchers interested in a grant should submit an informal Expression of Interest no later than March 31, 2023.
Requirements for the Expressions of Interest are as follows:
(1) A title for the project
(2) Basic information about the investigators. Please attach NIH biosketch.
(3) Basic hypothesis and background in no more than 400 words.
(4) Basic outline of the study, in no more than 1200 words. This must include:
(a) Toxicant(s) to be used. The study must involve a relatively common high-dose toxicant with demonstrated adverse impacts on germ cells. The common inhalational anesthetic sevoflurane would be a good example, at any stage of germline development. The common synthetic steroid pregnancy drug hydroxyprogesterone caproate, or 17-OHPC, would be a good example for a fetal exposure. Also, discuss the (physiologically relevant) dose(s) to be used and the controls. Please do not propose exposures that occur at low doses in the general population such as ambient endocrine-disrupting plasticizers or pesticides. Tobacco and smoking will also not be considered in this round.
(b) Mammal or human. The study must involve a mammalian model or a human cohort (eg, men undergoing or who have undergone inhalational general anesthesia for surgery). Explain the subjects of the study and the approximate "n."
(c) Developmental perspective. Explain the window(s) of germline development for your exposure, eg, embryonic, neonatal, childhood, adult, or near-conception exposure.
(d) Germline impact. The study must ascertain impacts on the exposed germ cells, and, if possible, the supporting somatic cells responsible for germ cell health and development. While the emphasis should be on impacts to germline epigenome, the endpoints can include:
—In males: any number of the following: genetic damage, epigenetic/chromatin alterations including impacts on imprinted genes, sperm concentration, morphology, motility; impacts on Sertoli cells. Explain whether the tissues to be tested are spermatogonial stem cells, mature spermatozoa, and/or other types.
—In females, any number of the following: genetic damage, epigenetic/chromatin alterations including impacts on imprinted genes, oocyte reserve, effects on other ovarian tissue.
Please explain the methods to be used to measure impacts.
(e) Optional — Heritable impact. Ascertainment of neurobehavioral outcomes in the generation born of the directly exposed cells. This would be "intergenerational" impacts — transgenerational impacts are outside the scope of this RFP.
(5) Brief description of funding requested (up to $30,000 per grant) and how the funds would be used, with line items. Due to the limited size of these grants, which are intended to generate pilot data on which larger grants may then be based, please reference possible leveraging of existing work or studies. If the work is to be done in phases, please describe those phases and the expected budgets for each. Note: For our grant programs, we have a policy to not pay indirect costs.
These informal expressions of interest are due March 31, 2023 and may be formatted as text in an email or as an attachment. Please email expressions of interest (questions prior to that are also welcome) to [email protected].
Authors of submissions meeting review criteria will be invited to submit more detailed information as may be needed. We reserve the right to make changes to this RFP at any time for any reason, and changes will be posted at germlineexposures.org/grants.
References:
Escher, Jill, Wei Yan, Emilie F. Rissman, Hsiao-Lin V. Wang, Arturo Hernandez, and Victor G. Corces. "Beyond genes: germline disruption in the etiology of autism spectrum disorders." Journal of Autism and Developmental Disorders 52, no. 10 (2022): 4608-4624.
Kaplanis, Joanna, Benjamin Ide, Rashesh Sanghvi, Matthew Neville, Petr Danecek, Tim Coorens, Elena Prigmore et al. "Genetic and chemotherapeutic influences on germline hypermutation." Nature 605, no. 7910 (2022): 503-508.
The Escher Fund for Autism is a donor-advised fund at Schwab Charitable. Questions may be directed to: [email protected]
• Investigation of heritable impacts preferred but not required
• Deadline: March 31, 2023
Background
Autism is a strongly heritable disorder that has, paradoxically, proven to be only weakly genetic, with rare genetic errors accounting for about 10-14% of cases. While many in the field now presume the "missing heritability" of autism can be found in common variants, The Escher Fund for Autism takes a different approach. We are concerned that much of the missing heritability of autism, along with much of its sharply increasing prevalence, is more likely to be found in the under-researched realm of germ cell toxicology.
In the modern world, human germ cells, egg and sperm and their precursors, do not arise in a pristine vacuum of biological perfection. Over the course of their development, our germ cells can suffer any number of toxicological challenges, which in some cases may reshape their molecular programming and ultimately their blueprint for gene expression in the offspring. While the vast majority of exposures likely have little to no effect, certain exposures in certain windows of vulnerability may cause pathology in the next generation, as demonstrated widely in mammalian models. Some of these exposures are discussed in Escher et al. 2022 (halogenated inhalational general anesthesia such as sevoflurane, smoking, synthetic steroids, HDAC inhibitors), with another example seen in Kaplanis et al. 2022 (chemotherapy drugs which lead to hypermutation in sperm).
Due to large gaps in knowledge in this field, this round of grants aims to encourage pilot studies investigating the impact of potent toxicants on male and/or female germ cells in mammalian models and/or human cohorts. While it is always preferable to investigate heritable impacts of germline exposures, the only required endpoints for this particular round of grants are the impacts on the germ cells (male and/or female) themselves.
Researchers interested in a grant should submit an informal Expression of Interest no later than March 31, 2023.
Requirements for the Expressions of Interest are as follows:
(1) A title for the project
(2) Basic information about the investigators. Please attach NIH biosketch.
(3) Basic hypothesis and background in no more than 400 words.
(4) Basic outline of the study, in no more than 1200 words. This must include:
(a) Toxicant(s) to be used. The study must involve a relatively common high-dose toxicant with demonstrated adverse impacts on germ cells. The common inhalational anesthetic sevoflurane would be a good example, at any stage of germline development. The common synthetic steroid pregnancy drug hydroxyprogesterone caproate, or 17-OHPC, would be a good example for a fetal exposure. Also, discuss the (physiologically relevant) dose(s) to be used and the controls. Please do not propose exposures that occur at low doses in the general population such as ambient endocrine-disrupting plasticizers or pesticides. Tobacco and smoking will also not be considered in this round.
(b) Mammal or human. The study must involve a mammalian model or a human cohort (eg, men undergoing or who have undergone inhalational general anesthesia for surgery). Explain the subjects of the study and the approximate "n."
(c) Developmental perspective. Explain the window(s) of germline development for your exposure, eg, embryonic, neonatal, childhood, adult, or near-conception exposure.
(d) Germline impact. The study must ascertain impacts on the exposed germ cells, and, if possible, the supporting somatic cells responsible for germ cell health and development. While the emphasis should be on impacts to germline epigenome, the endpoints can include:
—In males: any number of the following: genetic damage, epigenetic/chromatin alterations including impacts on imprinted genes, sperm concentration, morphology, motility; impacts on Sertoli cells. Explain whether the tissues to be tested are spermatogonial stem cells, mature spermatozoa, and/or other types.
—In females, any number of the following: genetic damage, epigenetic/chromatin alterations including impacts on imprinted genes, oocyte reserve, effects on other ovarian tissue.
Please explain the methods to be used to measure impacts.
(e) Optional — Heritable impact. Ascertainment of neurobehavioral outcomes in the generation born of the directly exposed cells. This would be "intergenerational" impacts — transgenerational impacts are outside the scope of this RFP.
(5) Brief description of funding requested (up to $30,000 per grant) and how the funds would be used, with line items. Due to the limited size of these grants, which are intended to generate pilot data on which larger grants may then be based, please reference possible leveraging of existing work or studies. If the work is to be done in phases, please describe those phases and the expected budgets for each. Note: For our grant programs, we have a policy to not pay indirect costs.
These informal expressions of interest are due March 31, 2023 and may be formatted as text in an email or as an attachment. Please email expressions of interest (questions prior to that are also welcome) to [email protected].
Authors of submissions meeting review criteria will be invited to submit more detailed information as may be needed. We reserve the right to make changes to this RFP at any time for any reason, and changes will be posted at germlineexposures.org/grants.
References:
Escher, Jill, Wei Yan, Emilie F. Rissman, Hsiao-Lin V. Wang, Arturo Hernandez, and Victor G. Corces. "Beyond genes: germline disruption in the etiology of autism spectrum disorders." Journal of Autism and Developmental Disorders 52, no. 10 (2022): 4608-4624.
Kaplanis, Joanna, Benjamin Ide, Rashesh Sanghvi, Matthew Neville, Petr Danecek, Tim Coorens, Elena Prigmore et al. "Genetic and chemotherapeutic influences on germline hypermutation." Nature 605, no. 7910 (2022): 503-508.
The Escher Fund for Autism is a donor-advised fund at Schwab Charitable. Questions may be directed to: [email protected]
2021 GRANT PROGRAMS [Archived]
• 2021 Grant Program #1: Investigation of heritable impacts of germ cell exposure to general anesthesia in experimental models or human cohorts
• 2021 Grant Program #2: Genetic and epigenetic impacts of general anesthesia on human sperm
|
$25,000 grants available, expressions of interest due March 31, 2021
2021 Grant Program #1: Investigation of heritable impacts of germ cell exposure to general anesthesia in experimental models or human cohorts Purpose:
Given the rapidly mounting evidence that agents of general anesthesia can impair germ cell integrity and result in neurodevelopmental abnormalities in offspring, our 2021 grant program will again focus on this urgent issue for public health. Studies in mammalian models have repeatedly demonstrated that germ cell exposure to agents of general anesthesia (GA) can cause adverse brain and behavior impacts in progeny borne of those germ cells via impacts on germline chromatin and epigenome. These findings are bolstered by a robust research literature demonstrating that GA agents, notably synthetic halogenated volatile general anesthetic gases, can impair the expression of genes crucial for early brain development. Given the observed adverse genomic, epigenomic and developmental effects of GA agents, combined with their ubiquitous use during all periods including sensitive periods of germ cell synthesis, it is concerning that little attention has been paid to potential links to germline contamination and heritable pathology. For a discussion of this phenomenon please see Escher J, Ford LD. General anesthesia, germ cells and the missing heritability of autism: an urgent need for research. Environ Epigen 2020;6:1, dvaa002. This set of grants aims to encourage both pilot studies in mammalian models and also retrospective studies in human cohorts. Applicants are encouraged to familiarize themselves with this literature, shown in rough chronological order:
Chalon J, et al. Exposure to halothane and enflurane affects learning function of murine progeny. Anesth Analg 1981;60:794–7. In a mouse model, learning retardation was seen in offspring of murine mothers exposed to GA in utero—in other words, mental impairment in the maternal line grandpups of the exposed gestating dams. Tang C-K, et al. Exposure of sires to enflurane affects learning function of murine progeny. Obstet Anesth Dig 1985;5(2):67. In a mouse model, the general anesthetic agent enflurane administered to male mice was found to adversely affected learning function of their offspring. Ju LS, et al. Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats. Brit J Anesth 2018;121(2);406-416. In a rat model, neonatal exposure to the widely used general anesthetic agent sevoflurane can affect the brains and behavior of the next generation of males through epigenetic modification of Kcc2 expression, while F1 females are at diminished risk. • Also See BJA editorial: Vutskits L, et al. A poisoned chalice: the heritage of parental anaesthesia exposure, Brit. J Anesth 2018. (“Hence, we are faced with a real possibility that general anaesthetics are not innocuous agents that ‘only put children to sleep’ but rather formidable modulators of chromatin remodeling and function…. The current study extends previous reports of sex differences by showing that anaesthetic exposure itself can alter expression of chloride channels in certain brain regions and that this effect is heritable from exposed male parents to unexposed offspring.”) Ju LS et al. Intergenerational Effects of Sevoflurane in Young Adult Rats. Anesthesiol 2019;131:1092-1109. Adult sevoflurane exposure affects brain development in male offspring by epigenetically reprograming both parental germ cells, while it induces neuroendocrine and behavioral abnormalities only in exposed males. Sex steroids may be required for mediation of the adverse effects of adult sevoflurane in exposed males. Chastain-Potts SE et al. Sevoflurane Exposure Results in Sex-Specific Transgenerational Upregulation of Target IEGs in the Subiculum. Mol Neurobiol 2019; https://doi.org/10.1007/s12035-019-01752-0. Neonatal female rats exposed to 6h of the general anesthesia gas sevoflurane had offspring whose brains exhibited epigenetic abnormalities, including reduced DNA methylation, an effect linked to functional decline in learning and memory. An upregulation of Arc and JunB mRNA expression, 71.6% and 74.0%, was seen in the male offspring. Also hypomethylation and modifications to IEGs crucial to synaptic plasticity were observed. The results suggest sevoflurane causes epigenetic modifications in the early rat oocytes. Martynyuk et al. Neuroendocrine, epigenetic, and intergenerational effects of general anesthetics. World J Psychiatr 2020; 10(5): 81-94. A review that includes a discussion of intergenerational effects. Escher J, Ford LD. General anesthesia, germ cells and the missing heritability of autism: an urgent need for research. Environ Epigen 2020;6:1, dvaa002. A commentary that stresses the urgency to research germline exposure to GA as a contributor to the missing heritability and increased prevalence of autism. Xu N, Lei L, Ju LS, Morey TE, Gravenstein N, Yang J, Martynyuk AE. A Methyltransferase Inhibitor (Decitabine) Alleviates Intergenerational Effects of Paternal Neonatal Exposure to Anesthesia With Sevoflurane. Anesth Analg 2020;131;4:1291-1299. Pepling M. Effects of In Utero Halothane Anesthesia Exposure on Germ Cell Methylation and Cognitive Behavior. Beyond Genes conference, presentation on unpublished data, December 11, 2020. Work in the Pepling lab shows halothane induces abnormal germ cell DNA methylation, and abnormal mouse offspring behaviors. Wang HLV. General Anesthetics Induce Epigenetic Alterations in Germ Cells that Result in Autism-like Behaviors. Beyond Genes conference, presentation on unpublished data, December 11, 2020. Work from the Corces lab shows how early sevoflurane causes abnormal TF binding in sperm, and abnormal brain and behavior of offspring, linked to dysregulation of autism-related genes. Preprint: Exposure to sevoflurane results in changes of transcription factor occupancy in sperm and inheritance of autism Xiao J, et al. Associations of parental birth characteristics with autism spectrum disorder (ASD) risk in their offspring: a population-based multigenerational cohort study in Denmark. Int J Epidemiol 2021;1-11, doi: 10.1093/ije/dyaa246. The first epidemiological study to investigate the question finds parental prematurity is linked to offspring autism risk, raising questions about germ cell epigenetics. It is worth noting that premature infants have much higher rates of surgery and exposure to anesthetic agents, opiates, and corticosteroids than full term infants. Requirements for Expressions of Interest
Expressions of interest must concern either (1) a mammalian model of GA exposure to germ cells, with ascertainment of neurobehavioral outcomes in the generation born of the exposed cells, or (2) a human retrospective study where two points of data may be ascertained: (a) a type of parent exposure to surgery (for example, certain types of neonatal surgeries across a certain timeframe), and controls, and (b) neurobehavioral outcomes in offspring. Proposals must include the following: (1) A title for the project and basic information about the investigators. Please attach NIH biosketch. (2) Basic hypothesis and approach. Must include information about: (a) The timing and intensity of exposure For experimental models, please explain the developmental window(s) being considered, eg: • Exposure to gestating dam (that is, fetal germ cells) • Exposure to neonates • Exposure before sexual maturity • Exposure after sexual maturity For human studies, please explain the window(s) of exposure being considered, eg: • Embryonic and fetal (and minor procedures v major procedures) • Perinatal/neonatal (and minor procedures v major procedures) • Early childhood (eg, ages 1-5) (and minor procedures v major procedures) • Adolescence (eg, ages 9-16) (and minor procedures v major procedures) • Preconception (eg, for males within 2-3 months of conception) (and minor procedures v major procedures) • And/or rough "Composite score of parental surgery under GA." Categories of exposure could include, for example, 0 surgery; 1 minor surgery (less than an hour); 2-3 minor surgeries; 4+ surgeries. Because developmental stage is crucial there would need to be timing parameters as well. (b) The nature of the exposure For experimental models, please explain the agent(s) being used. Inhalation anesthetics may include any single or combination of nitrous oxide, halothane, enflurane, isoflurane, desflurane, and/or sevoflurane. Intravenous or other anesthetics may be used in combination. For human cohorts, please note that GA agents and doses used are often omitted from medical records. Surgery of a certain type, duration, or complexity that typically entails GA will likely need to be used as a proxy for GA exposure. (c) The approximate "n" of subjects For experimental models, please explain the approximate number of animals involved, including the exposed generation and the offspring generation. For human cohorts, please explain the approximate n of exposed parent generation and controls, and "n" of offspring, that may be ascertained. (d) Offspring neurodevelopmental outcome For experimental models, please explain how behavioral, learning, socio-sexual, and/or neurodevelopmental outcomes will be ascertained. For human cohorts, please explain how subjects' offspring neurodevelopmental outcomes may be ascertained, with attention to differential sex effects. Outcomes may include, for example: autism, learning disability, intellectual disability, ADHD, anxiety, motor impairments, vision impairments, behavioral impairments, etc. (e) Measurements of DNA damage and/or gene expression For experimental models, please explain how the study would measure epigenetic or genetic changes in the exposed germ cells, and/or in the brain tissues of the offspring born of the exposed cells. [This requirement does not apply to human studies. 2021 Grant Program #2 addresses this question in human males.] (3) Brief description of funding requested and how the funds would be used. Due to the limited size of these grants, please reference possible leveraging of data or work from existing studies. If the work is to be done in phases, please describe those phases and the expected budgets for each. How to apply Informal expressions of interest are due March 31, 2021 and should be no longer than three pages and may be formatted as text in an email rather than an attachment. Please email expressions of interest (questions prior to that are welcome) to [email protected]. Authors of submissions meeting review criteria will be invited to submit more detailed applications. We reserve the right to make changes to this RFP at any time for any reason, and changes will be posted at germlineexposures.org/grants. RFP posted: January 15, 2021. The Escher Fund for Autism is a donor-advised fund at Schwab Charitable. Note: For our grant programs, we have a policy to not pay indirect costs. $25,000 grants available, expressions of interest due March 31, 2021 [Archived]
2021 Grant Program #2: Genetic and epigenetic impacts of general anesthesia on human sperm For general background on the topic of heritable impacts of GA, please see 2021 Grant Program #1, above.
Purpose To ascertain potential deleterious impacts of general anesthesia on the quality of human sperm, with an emphasis on DNA damage and epigenetic impacts. Requirements for Expressions of Interest Expressions of interest must concern ascertainment of changes in human sperm in subjects who have undergone general anesthesia. Proposals must include the following: (1) A title for the project and basic information about the investigators. Please attach NIH biosketch. (2) Basic hypothesis and approach. Must include information about: (a) The nature of the exposure For example, a study of men who have undergone certain types of surgical procedures, or procedures of a certain duration. Preferably the study would include collecting samples before and after the procedures. Please indicate if it would be possible to gather information on the anesthetic agents used, and at what concentrations, for what durations. In addition, we would consider a study based entirely on exposures in the past, even the distant past, with no "before" collections. For example a study of sperm qualities in males who had undergone numerous inpatient surgeries over their lifetimes v males who had undergone no surgeries. Composite lifetime exposure scores, or exposures restricted to certain germ cell developmental windows will be considered. (b) The approximate "n" of subjects For human cohorts, please explain the approximate n of exposed parent generation and controls, and "n" of offspring, that may be ascertained. (c) Measurements of DNA damage and/or epigenetic perturbations Please explain how the study would measure epigenetic or genetic changes in the sperm, and at what timepoint(s). Measurement of additional parameters are strongly suggested as well, including morphology, motility, concentration. (3) Brief description of funding requested and how the funds would be used. Due to the limited size of these grants, please reference possible leveraging of data or work from existing studies. If the work is to be done in phases, please describe those phases and the expected budgets for each. How to apply Informal expressions of interest are due March 31, 2021 and should be no longer than three pages and may be formatted as text in an email rather than an attachment. Please email expressions of interest (questions prior to that are welcome) to [email protected]. Authors of submissions meeting review criteria will be invited to submit more detailed applications. We reserve the right to make changes to this RFP at any time for any reason, and changes will be posted at germlineexposures.org/grants. RFP posted: January 15, 2021. The Escher Fund for Autism is a donor-advised fund at Schwab Charitable. Note: For our grant programs, we have a policy to not pay indirect costs. |
$25,000 grants available, expressions of interest due May 31, 2020
Investigation of heritable impacts of germ cell exposure to general anesthesia in human cohorts
Investigation of heritable impacts of germ cell exposure to general anesthesia in human cohorts
Studies in mammal models have repeatedly demonstrated that germ cell exposure to agents of general anesthesia (GA) can cause adverse brain and behavior impacts in progeny borne of those germ cells, likely via impacts on germline chromatin and epigenome. These findings are bolstered by a robust research literature demonstrating that GA agents, notably synthetic halogenated volatile general anesthetic gases, can impair the expression of genes crucial for early brain development.
However, to date no studies on heritable impacts of GA have been performed in human cohorts. This set of grants aims to encourage pilot retrospective studies that begin to fill this important knowledge gap.
However, to date no studies on heritable impacts of GA have been performed in human cohorts. This set of grants aims to encourage pilot retrospective studies that begin to fill this important knowledge gap.
The five mammal studies published so far:
Chastain-Potts SE et al. Sevoflurane Exposure Results in Sex-Specific Transgenerational Upregulation of Target IEGs in the Subiculum. Mol Neurobiol 2019; https://doi.org/10.1007/s12035-019-01752-0.
Neonatal female rats exposed to 6h of the general anesthesia gas sevoflurane had offspring whose brains exhibited epigenetic abnormalities, including reduced DNA methylation, an effect linked to functional decline in learning and memory. An upregulation of Arc and JunB mRNA expression, 71.6% and 74.0%, was seen in the male offspring. Also hypomethylation and modifications to IEGs crucial to synaptic plasticity were observed. The results suggest sevoflurane causes epigenetic modifications in the early rat oocytes.
Ju LS et al. Intergenerational Effects of Sevoflurane in Young Adult Rats. Anesthesiol 2019;131:1092-1109.
Adult sevoflurane exposure affects brain development in male offspring by epigenetically reprograming both parental germ cells, while it induces neuroendocrine and behavioral abnormalities only in exposed males. Sex steroids may be required for mediation of the adverse effects of adult sevoflurane in exposed males.
Ju LS et al. Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats. Brit J Anesth 2018;121(2);406-416.
In a rat model, neonatal exposure to the widely used general anesthetic agent sevoflurane can affect the brains and behavior of the next generation of males through epigenetic modification of Kcc2 expression, while F1 females are at diminished risk.
• Also See BJA editorial: Vutskits L, et al. A poisoned chalice: the heritage of parental anaesthesia exposure, Brit. J Anesth 2018. (“Hence, we are faced with a real possibility that general anaesthetics are not innocuous agents that ‘only put children to sleep’ but rather formidable modulators of chromatin remodeling and function…. The current study extends previous reports of sex differences by showing that anaesthetic exposure itself can alter expression of chloride channels in certain brain regions and that this effect is heritable from exposed male parents to unexposed offspring.”)
Chalon J, et al. Exposure to halothane and enflurane affects learning function of murine progeny. Anesth Analg 1981;60:794–7.
In a mouse model, learning retardation was seen in offspring of murine mothers exposed to GA in utero—in other words, mental impairment in the maternal line grandpups of the exposed gestating dams.
Tang C-K, et al. Exposure of sires to enflurane affects learning function of murine progeny. Obstet Anesth Dig 1985;5(2):67.
In a mouse model, the general anesthetic agent enflurane administered to male mice was found to adversely affected learning function of their offspring.
Given the observed adverse genomic, epigenomic and developmental effects of GA agents, combined with their fairly common use during gestational, neonatal and pre-conception periods, it is concerning that little attention has been paid to potential links to germline contamination and heritable pathology.
Chastain-Potts SE et al. Sevoflurane Exposure Results in Sex-Specific Transgenerational Upregulation of Target IEGs in the Subiculum. Mol Neurobiol 2019; https://doi.org/10.1007/s12035-019-01752-0.
Neonatal female rats exposed to 6h of the general anesthesia gas sevoflurane had offspring whose brains exhibited epigenetic abnormalities, including reduced DNA methylation, an effect linked to functional decline in learning and memory. An upregulation of Arc and JunB mRNA expression, 71.6% and 74.0%, was seen in the male offspring. Also hypomethylation and modifications to IEGs crucial to synaptic plasticity were observed. The results suggest sevoflurane causes epigenetic modifications in the early rat oocytes.
Ju LS et al. Intergenerational Effects of Sevoflurane in Young Adult Rats. Anesthesiol 2019;131:1092-1109.
Adult sevoflurane exposure affects brain development in male offspring by epigenetically reprograming both parental germ cells, while it induces neuroendocrine and behavioral abnormalities only in exposed males. Sex steroids may be required for mediation of the adverse effects of adult sevoflurane in exposed males.
Ju LS et al. Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats. Brit J Anesth 2018;121(2);406-416.
In a rat model, neonatal exposure to the widely used general anesthetic agent sevoflurane can affect the brains and behavior of the next generation of males through epigenetic modification of Kcc2 expression, while F1 females are at diminished risk.
• Also See BJA editorial: Vutskits L, et al. A poisoned chalice: the heritage of parental anaesthesia exposure, Brit. J Anesth 2018. (“Hence, we are faced with a real possibility that general anaesthetics are not innocuous agents that ‘only put children to sleep’ but rather formidable modulators of chromatin remodeling and function…. The current study extends previous reports of sex differences by showing that anaesthetic exposure itself can alter expression of chloride channels in certain brain regions and that this effect is heritable from exposed male parents to unexposed offspring.”)
Chalon J, et al. Exposure to halothane and enflurane affects learning function of murine progeny. Anesth Analg 1981;60:794–7.
In a mouse model, learning retardation was seen in offspring of murine mothers exposed to GA in utero—in other words, mental impairment in the maternal line grandpups of the exposed gestating dams.
Tang C-K, et al. Exposure of sires to enflurane affects learning function of murine progeny. Obstet Anesth Dig 1985;5(2):67.
In a mouse model, the general anesthetic agent enflurane administered to male mice was found to adversely affected learning function of their offspring.
Given the observed adverse genomic, epigenomic and developmental effects of GA agents, combined with their fairly common use during gestational, neonatal and pre-conception periods, it is concerning that little attention has been paid to potential links to germline contamination and heritable pathology.
For more details regarding this hypothesis and genetic/epigenetic toxicity of GA generally, please see Escher Fund work here:
• NIEHS Environmental Factor Newsletter [November 2019 article]
• Heritable Impacts of Toxicant Exposures: A Gap in Research and Regulation [NIEHS, September 2019, video]
• Germ Cell Exposure to Toxicants: A Gap in Research and Regulation [Environmental Mutagenesis and Genomics Society, September 2019, video]
• Heritable Hazards of General Anesthesia [Epigenetic Inheritance Symposium, Zurich August 2019, video]
• Germ Cell Toxicant Exposure: A Gap in Research and Regulation [Brazil Mutagenesis Conference, June 2019, video]
• NIH research program on heritable (germline) impacts of general anesthesia, a response to Dr. Bianchi’s November 27, 2018 letter [June 2019 letter]
• Pregnancy Drugs, Fetal Germline Epigenome, and Risks for Next-Generation Pathology: A Call to Action. By Jill Escher and Suzanne Robotti [2019 Environmental and Molecular Mutagenesis journal]
• Bugs in the Program: why non-genetic inheritance should be a priority in autism research. By Jill Escher [May 2018 Environmental Epigenetics journal]
• "Quasi-Genetics" at the Roots of Autism? By LaDonna Ford, MD and Jill Escher
[2019 blogpost]
• 120+ studies demonstrating nongenetic inheritance in mammals and humans
• Nomination of germ cell impacts of general anesthetic gases to National Toxicology Program [November 2018]
• Nomination for OHAT scoping review of human and mammal evidence for adverse heritable impacts of direct germ cell exposure to drugs and chemicals [December 2018]
Requirements for Expressions of Interest
Expressions of interest must concern a human retrospective study, where two points of data may be ascertained: (1) a type of parent exposure to surgery (for example, certain types of neonatal surgeries across a certain timeframe), and controls, and (2) neurobehavioral outcomes in offspring. Proposals must include the following:
(1) A title for the project and basic information about the investigators. Please attach NIH biosketch.
(2) Basic hypothesis and approach. Must include information about:
(a) The timing and intensity of exposure. For example:
• Embryonic and fetal (and minor procedures v major procedures)
• Perinatal/neonatal (and minor procedures v major procedures)
• Early childhood (eg, ages 1-5) (and minor procedures v major procedures)
• Adolescence (eg, ages 9-16) (and minor procedures v major procedures)
• Preconception (eg, for males within 2-3 months of conception) (and minor procedures v major procedures)
• And/or rough "Composite score of parental surgery under GA." Categories of exposure could include, for example, 0 surgery; 1 minor surgery (less than an hour); 2-3 minor surgeries; 4+ surgeries. Because developmental stage is crucial there would need to be timing parameters as well.
(b) The nature of the exposure. GA agents and doses used are often omitted from medical records. Surgery of a certain type, duration, or complexity that typically entails GA will likely need to be used as a proxy for GA exposure.
(c) The approximate "n" of subjects (exposed parent generation) and controls, and "n" of offspring, that may be ascertained. Big data and composite "surgery exposure scores" within certain germ cell developmental windows will be considered.
(d) The cohort and how subjects' offspring neurodevelopmental outcomes may be ascertained, with attention to differential sex effects. Outcomes may include, for example: autism, learning disability, intellectual disability, ADHD, anxiety, motor impairments, vision impairments, behavioral impairments, etc.
(3) Brief description of funding requested and how the funds would be used. Due to the limited size of these grants, please reference possible leveraging of data or work from existing studies. If the work is to be done in phases, please describe those phases and the expected budgets for each.
Also — please indicate how you expect the research can be conducted given the current constraints of the pandemic.
How to apply
Informal expressions of interest are due May 31, 2020 and should be no longer than three pages and may be formatted as text in an email rather than an attachment. Please email expressions of interest (questions prior to that are welcome) to [email protected].
Authors of submissions meeting review criteria will be invited to submit more detailed applications. We reserve the right to make changes to this RFP at any time for any reason, and changes will be posted at germlineexposures.org/grants.
RFP posted: January 10, 2020.
The Escher Fund for Autism is a donor-advised fund at Schwab Charitable.
• NIEHS Environmental Factor Newsletter [November 2019 article]
• Heritable Impacts of Toxicant Exposures: A Gap in Research and Regulation [NIEHS, September 2019, video]
• Germ Cell Exposure to Toxicants: A Gap in Research and Regulation [Environmental Mutagenesis and Genomics Society, September 2019, video]
• Heritable Hazards of General Anesthesia [Epigenetic Inheritance Symposium, Zurich August 2019, video]
• Germ Cell Toxicant Exposure: A Gap in Research and Regulation [Brazil Mutagenesis Conference, June 2019, video]
• NIH research program on heritable (germline) impacts of general anesthesia, a response to Dr. Bianchi’s November 27, 2018 letter [June 2019 letter]
• Pregnancy Drugs, Fetal Germline Epigenome, and Risks for Next-Generation Pathology: A Call to Action. By Jill Escher and Suzanne Robotti [2019 Environmental and Molecular Mutagenesis journal]
• Bugs in the Program: why non-genetic inheritance should be a priority in autism research. By Jill Escher [May 2018 Environmental Epigenetics journal]
• "Quasi-Genetics" at the Roots of Autism? By LaDonna Ford, MD and Jill Escher
[2019 blogpost]
• 120+ studies demonstrating nongenetic inheritance in mammals and humans
• Nomination of germ cell impacts of general anesthetic gases to National Toxicology Program [November 2018]
• Nomination for OHAT scoping review of human and mammal evidence for adverse heritable impacts of direct germ cell exposure to drugs and chemicals [December 2018]
Requirements for Expressions of Interest
Expressions of interest must concern a human retrospective study, where two points of data may be ascertained: (1) a type of parent exposure to surgery (for example, certain types of neonatal surgeries across a certain timeframe), and controls, and (2) neurobehavioral outcomes in offspring. Proposals must include the following:
(1) A title for the project and basic information about the investigators. Please attach NIH biosketch.
(2) Basic hypothesis and approach. Must include information about:
(a) The timing and intensity of exposure. For example:
• Embryonic and fetal (and minor procedures v major procedures)
• Perinatal/neonatal (and minor procedures v major procedures)
• Early childhood (eg, ages 1-5) (and minor procedures v major procedures)
• Adolescence (eg, ages 9-16) (and minor procedures v major procedures)
• Preconception (eg, for males within 2-3 months of conception) (and minor procedures v major procedures)
• And/or rough "Composite score of parental surgery under GA." Categories of exposure could include, for example, 0 surgery; 1 minor surgery (less than an hour); 2-3 minor surgeries; 4+ surgeries. Because developmental stage is crucial there would need to be timing parameters as well.
(b) The nature of the exposure. GA agents and doses used are often omitted from medical records. Surgery of a certain type, duration, or complexity that typically entails GA will likely need to be used as a proxy for GA exposure.
(c) The approximate "n" of subjects (exposed parent generation) and controls, and "n" of offspring, that may be ascertained. Big data and composite "surgery exposure scores" within certain germ cell developmental windows will be considered.
(d) The cohort and how subjects' offspring neurodevelopmental outcomes may be ascertained, with attention to differential sex effects. Outcomes may include, for example: autism, learning disability, intellectual disability, ADHD, anxiety, motor impairments, vision impairments, behavioral impairments, etc.
(3) Brief description of funding requested and how the funds would be used. Due to the limited size of these grants, please reference possible leveraging of data or work from existing studies. If the work is to be done in phases, please describe those phases and the expected budgets for each.
Also — please indicate how you expect the research can be conducted given the current constraints of the pandemic.
How to apply
Informal expressions of interest are due May 31, 2020 and should be no longer than three pages and may be formatted as text in an email rather than an attachment. Please email expressions of interest (questions prior to that are welcome) to [email protected].
Authors of submissions meeting review criteria will be invited to submit more detailed applications. We reserve the right to make changes to this RFP at any time for any reason, and changes will be posted at germlineexposures.org/grants.
RFP posted: January 10, 2020.
The Escher Fund for Autism is a donor-advised fund at Schwab Charitable.
2020 Germ Cell Exposure Mini-Grants

We are happy to announce a new round of mini-grants, from $250 to $5,000..
Grants are limited — without exception — to projects related to adverse heritable impacts of germ cell exposures to pharmaceuticals, drugs, or chemicals (such as tobacco, general anesthesia, psychoactive drugs, synthetic steroids, some low-dose "environmental" exposures will also be considered). Phenotypic endpoints must relate to brain and/or behavior of offspring borne of exposed germline (sorry, metabolism, obesity, and cancer friends, but maybe next year). Human or mammal work is strongly preferred (sorry, fish, fly and worm friends).
Please do not submit anything relating to somatic exposures or pure genetics, your application will be rejected.
Examples of Escher Fund mini-grants from past years:
--Support for symposia, conferences, meetings and travel
--Expenses associated with publishing a new study, review or commentary
--Excess costs in performing an ongoing study
To apply, simply email us [email protected] with your name, affiliation, contact info (including link to lab), brief description of overall project, specific purpose of the grant, and amount requested. We will respond with any questions.
WE REGRET THAT THIS PROGRAM IS CURRENTLY ON HOLD. RESUMPTION WILL BE ANNOUNCED IN OUR NEWSLETTER.
Grants are limited — without exception — to projects related to adverse heritable impacts of germ cell exposures to pharmaceuticals, drugs, or chemicals (such as tobacco, general anesthesia, psychoactive drugs, synthetic steroids, some low-dose "environmental" exposures will also be considered). Phenotypic endpoints must relate to brain and/or behavior of offspring borne of exposed germline (sorry, metabolism, obesity, and cancer friends, but maybe next year). Human or mammal work is strongly preferred (sorry, fish, fly and worm friends).
Please do not submit anything relating to somatic exposures or pure genetics, your application will be rejected.
Examples of Escher Fund mini-grants from past years:
--Support for symposia, conferences, meetings and travel
--Expenses associated with publishing a new study, review or commentary
--Excess costs in performing an ongoing study
To apply, simply email us [email protected] with your name, affiliation, contact info (including link to lab), brief description of overall project, specific purpose of the grant, and amount requested. We will respond with any questions.
WE REGRET THAT THIS PROGRAM IS CURRENTLY ON HOLD. RESUMPTION WILL BE ANNOUNCED IN OUR NEWSLETTER.
Third 2018 Grant Opportunity — $25k grant available, expressions of interest due October 31, 2018
Investigation of F2 neurodevelopmental outcomes of F0 pregnancy or F1 neonatal general anesthesia
Investigation of F2 neurodevelopmental outcomes of F0 pregnancy or F1 neonatal general anesthesia
$25k RFP: Letters of interest due October 31, 2018
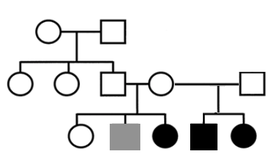
Consider these cases--
These family histories, gathered during informal autism parent interviews, raise the question: can exposure to an agent of general anesthesia (GA) affect early germline programming (proto-sperm or proto-egg), causing dysregulation of early neurodevelopment in offspring borne of the affected gamete? A striking new study on rats suggests the answer may be yes.
The study by Ju et al, Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats, found neonatal exposure to sevoflurane can affect the next generation of males via germline mediated epigenetic mechanisms, while females were at diminished risk. The study is not the first animal model to observe a next-generation effect of GA. In 1981, Chalon et al found learning retardation in offspring of murine parents exposed to GA in utero—in other words, mental impairment in the grandpups of the exposed gestating dams. See Exposure to halothane and enflurane affects learning function of murine progeny. And in 1984 the same lab noted that alterations to reproductive cells of adult male mice via the GA agent enflurane adversely affected learning function of their offspring. See Exposure of sires to enflurane affects learning function of murine progeny.
Given the known adverse genomic, epigenomic and developmental effects of GA agents, combined with their fairly common use during gestational and neonatal periods, it is concerning that little attention has been paid to potential links to germline contamination and heritable pathology. This RFP is intended to help explicitly address this gap in the research by prompting a pilot study in a human cohort or an animal model (human study is preferable). For more information on this hypothesis, see Bugs in the program: can pregnancy drugs and smoking disturb molecular reprogramming of the fetal germline, increasing heritable risk for autism and neurodevelopmental disorders? in Environmental Epigenetics, observing that autism family histories seemed to point to prenatal or neonatal GA as a potent germline toxicant.
Proposal requirements
Must include the following:
(1) A title for the project and basic information about the investigators. Please attach NIH biosketch.
(2) Basic hypothesis.
(3) Brief description of the proposed methodology.
(4) Identify the GA exposure(s) of interest. This could include any common GA agent(s) used from the period 1950 to today, for example halothane, enflurane, desfluane, sevoflurane. Note that In human cohorts, GA agents and doses used are often omitted from medical records. Surgery that typically entails GA may be used as a proxy for GA exposure, though the precise identity of the chemicals used may be unknown.
(5) Timing of exposure. Exposure must be in time frames between the primordial germ cell (embryonic-fetal) period through through infancy.
(6) Neurodevelopmental outcomes in the offspring of the exposed germ cells to be ascertained, with attention to differential sex effects. Outcomes may include, for example: autism, learning disability, intellectual disability, ADHD, anxiety, motor impairments, vision impairments. [If an animal model is proposed, molecular investigations of germ cells, brain cells, or other tissue is encouraged, but optional given the limited size of the grant.]
(7) An idea about mechanism. For example, the recent editorial, "A poisoned chalice: the heritage of parental anaesthesia exposure," discussed the idea that key transcription factors induced in the germ cell could up- or down-regulate target genes via histone modification, and/or modification of DNA methylation. "Hence, we are faced with a real possibility that general anaesthetics are not innocuous agents that ‘only put children to sleep’ but rather formidable modulators of chromatin remodelling and function," the authors stated. This part need not be lengthy, it is intended merely to suggest a biological plausibility of the hypothesis.
(8) Description of how the funds (up to $25k) would be used. Due to the limited size of this grant, please reference possible leveraging of data or work from existing studies. If the work is to be done in phases, please describe those phases and the expected budgets for each.
How to apply
Informal expressions of interest are due October 31, 2018, and should be no longer than three pages and can be in formatted as text in an email rather than an attachment. Please email expressions of interest (questions prior to that are welcome) to [email protected].
Authors of submissions meeting review criteria will be invited to submit more detailed applications in the following months. We reserve the right to make changes to this RFP at any time for any reason, and changes will be posted at germlineexposures.org/grants.
RFP posted: June 21, 2018, revised August 31, September 4, and September 28, 2018.
The Escher Fund for Autism is a donor-advised fund at Schwab Charitable.
Consider these cases--
- Mother has two daughters with idiopathic autism. She had been in utero when her mother had surgery following appendicitis.
- Father has a son with idiopathic autism. He had been in utero when his mother underwent surgery to fix a problem with the placenta.
- Mother has three children with idiopathic autism. She was in utero when her mother had surgery following an accident.
- Mother has two boys with idiopathic autism. She had been born with a spinal tumor and hernia, and had undergone two surgeries in infancy.
- Mother has one son with idiopathic autism. She had been born with a cleft palate (presumably had early surgery, or series of surgeries, though details are unknown).
- Mother has two sons with idiopathic autism. She had been born with a heart defect (presumably had early surgery, or series of surgeries, though details are unknown).
- Father has three children with idiopathic autism. He had been born with a significant birth defect affecting his foot (presumably had early surgery, or series of surgeries, though details are unknown).
These family histories, gathered during informal autism parent interviews, raise the question: can exposure to an agent of general anesthesia (GA) affect early germline programming (proto-sperm or proto-egg), causing dysregulation of early neurodevelopment in offspring borne of the affected gamete? A striking new study on rats suggests the answer may be yes.
The study by Ju et al, Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats, found neonatal exposure to sevoflurane can affect the next generation of males via germline mediated epigenetic mechanisms, while females were at diminished risk. The study is not the first animal model to observe a next-generation effect of GA. In 1981, Chalon et al found learning retardation in offspring of murine parents exposed to GA in utero—in other words, mental impairment in the grandpups of the exposed gestating dams. See Exposure to halothane and enflurane affects learning function of murine progeny. And in 1984 the same lab noted that alterations to reproductive cells of adult male mice via the GA agent enflurane adversely affected learning function of their offspring. See Exposure of sires to enflurane affects learning function of murine progeny.
Given the known adverse genomic, epigenomic and developmental effects of GA agents, combined with their fairly common use during gestational and neonatal periods, it is concerning that little attention has been paid to potential links to germline contamination and heritable pathology. This RFP is intended to help explicitly address this gap in the research by prompting a pilot study in a human cohort or an animal model (human study is preferable). For more information on this hypothesis, see Bugs in the program: can pregnancy drugs and smoking disturb molecular reprogramming of the fetal germline, increasing heritable risk for autism and neurodevelopmental disorders? in Environmental Epigenetics, observing that autism family histories seemed to point to prenatal or neonatal GA as a potent germline toxicant.
Proposal requirements
Must include the following:
(1) A title for the project and basic information about the investigators. Please attach NIH biosketch.
(2) Basic hypothesis.
(3) Brief description of the proposed methodology.
(4) Identify the GA exposure(s) of interest. This could include any common GA agent(s) used from the period 1950 to today, for example halothane, enflurane, desfluane, sevoflurane. Note that In human cohorts, GA agents and doses used are often omitted from medical records. Surgery that typically entails GA may be used as a proxy for GA exposure, though the precise identity of the chemicals used may be unknown.
(5) Timing of exposure. Exposure must be in time frames between the primordial germ cell (embryonic-fetal) period through through infancy.
(6) Neurodevelopmental outcomes in the offspring of the exposed germ cells to be ascertained, with attention to differential sex effects. Outcomes may include, for example: autism, learning disability, intellectual disability, ADHD, anxiety, motor impairments, vision impairments. [If an animal model is proposed, molecular investigations of germ cells, brain cells, or other tissue is encouraged, but optional given the limited size of the grant.]
(7) An idea about mechanism. For example, the recent editorial, "A poisoned chalice: the heritage of parental anaesthesia exposure," discussed the idea that key transcription factors induced in the germ cell could up- or down-regulate target genes via histone modification, and/or modification of DNA methylation. "Hence, we are faced with a real possibility that general anaesthetics are not innocuous agents that ‘only put children to sleep’ but rather formidable modulators of chromatin remodelling and function," the authors stated. This part need not be lengthy, it is intended merely to suggest a biological plausibility of the hypothesis.
(8) Description of how the funds (up to $25k) would be used. Due to the limited size of this grant, please reference possible leveraging of data or work from existing studies. If the work is to be done in phases, please describe those phases and the expected budgets for each.
How to apply
Informal expressions of interest are due October 31, 2018, and should be no longer than three pages and can be in formatted as text in an email rather than an attachment. Please email expressions of interest (questions prior to that are welcome) to [email protected].
Authors of submissions meeting review criteria will be invited to submit more detailed applications in the following months. We reserve the right to make changes to this RFP at any time for any reason, and changes will be posted at germlineexposures.org/grants.
RFP posted: June 21, 2018, revised August 31, September 4, and September 28, 2018.
The Escher Fund for Autism is a donor-advised fund at Schwab Charitable.
ARCHIVE ONLY. THIS GRANT ROUND HAS BEEN PROCESSED.
Second 2018 Grant Opportunity — $25k grant available, expressions of interest due June 30, 2018
Second 2018 Grant Opportunity — $25k grant available, expressions of interest due June 30, 2018
Examining molecular impacts of drug exposures on developing germline, and implications for nongenetic inheritance
Drugs have been used in abundance in pregnancy since the 1950s. While somatic impacts have received attention, very little research has examined potential molecular impact on the germline.
There has been growing concern about adverse impacts of environmental chemicals such as plasticizers, pesticides and fungicides on embryonic and fetal germline. However, a greater threat to molecular integrity of our cells of inheritance may stem from a nearly unstudied realm of exposure — the heavy and continual doses of chemicals introduced into the gestating female in the form of prescription, over-the-counter and recreational drugs.
Despite the widespread use of pregnancy drugs since the 1950s and the well established molecular vulnerability of the early germline, little research has been conducted on this interface, and in fact the US Food and Drug Administration has refused to consider embryonic and fetal germline as a tissue of interest in safety assessment of pregnancy drugs it regulates. The FDA has refused to consider early germ cell risks even when petitioned to do so (see, for example, Escher Fund for Autism petitions linked here and here). Likewise, the FDA's tobacco program does not include attention to the vulnerability of germ cells to tobacco exposure (see, for example, Escher Fund letter to FDA here).
This RFP is intended to help address this gap in the research by encouraging projects that directly focus on early (primordial germ cell in embryo through neonatal period) germline molecular impacts of high and sustained dose pregnancy drugs. Perturbations of early molecular programming may yield outsize effects, holding the key to understanding the heritable etiology of a subset of developmental pathologies.
Informal expressions of interest in this grant are due June 30, 2018, and should include the following:
(1) A title for the project and basic information about the investigators.
(2) Hypothesis. What are you proposing to look at and why? Please discuss what exogenous substance(s) are to be tested and why they may be of concern. Please state if the substance(s) are hormone disruptors or known to have genotoxic effects. Please state what molecular mechanisms might be involved. For example, might the substance(s) bind to certain receptors and activate transcription in the germ cells, affecting how the DNA gets re-methylated in the germ cells and after fertilization, thereby causing changes in transcription in adult tissues? Through any mechanisms might the substance(s) up or down-regulate genes known to be essential for proper neurodevelopment? Does the substance weaken protective marks, precipitating increased risk for de novo mutagenesis?
Regarding substances of interest, these could include drugs common in the past, for example those used during the post-war pregnancy drug boom, or commonly used today, either for medical or recreational purposes.
Common during the post-war pregnancy drug boom (these are examples only):
- Synthetic steroid hormones, such as diethylstilbestrol, other synthetic estrogens, progestins such as 17-OHPC, and corticosteroids (see Prenatal Exposure to Synthetic Progestins and Estrogens: Effects on Human Development for an overview of hormone mimics used in the post-war decades).
- Barbiturates ("Between 1950 and the late 1970s more than 22 million children were born in the United
States to women taking prescribed barbiturates during pregnancy" see here) - Amphetamines (for several decades prescribed for weight control in pregnancy, see eg this 1949 paper)
- Diuretics (see Diuretics in pregnancy: a case study of a worthless therapy)
- General anesthesic agents used in the past (GA agents have been show to cause changes in chromatin in brain neurons, see eg here)
- Analgesics
- Tobacco (known to contain mutagenic and epimutagenic components, see eg here)
Common today (these are examples only):
- The synthetic progestin 17-OHPC, marketed at Makena, is today widely administered during gestational weeks 16-32 for ostensible prevention of preterm birth. (See 17-alpha Hydroxyprogesterone caproate did not reduce the rate of recurrent preterm birth in a prospective cohort study)
- Doxylamine succinate and pyridoxine hydrochloride, marketed as Diclegis, is widely used through week 12 as a morning sickness medication.
- Antidepressants (for example SSRI's), used in up to 6% of US pregnancies.
- Antipsychotics, and other psychoactive drugs.
- Analgesics (for example acetominophen, ibuprofen)
- Opioids (for example OxyContin)
- General anesthetic agents
- Tobacco
In spite of the low budget offered via this RFP, a study that examines effects of more than one substance is preferred.
(3) Brief description of the proposed methodology (eg, animal model, in vitro), including description of molecular mechanisms, gene expression patterns, and/or phenotypes to be observed. There must also be attention paid to potential adverse developmental (particularly neurodevelopmental and behavioral) consequences of any observed perturbations.
(4) Description of how the funds (up to $25k) would be used. Due to the limited size of this grant, please reference possible leveraging of data or work from existing studies. If the work is to be done in phases, please describe those phases and the expected budgets for each.
How to apply
Informal expressions of interest are due no later than June 30, 2018. The document should be no longer than three pages and can be in formatted as text in an email rather than an attachment. Please email expressions of interest (questions prior to that are welcome) to [email protected].
Authors of submissions meeting review criteria will be invited to submit more detailed applications in the following months. We reserve the right to make changes to this RFP at any time for any reason, and changes will be posted at germlineexposures.org/grants.
Date posted: February 27, 2018.
The Escher Fund for Autism is a donor-advised fund at Schwab Charitable.
ARCHIVE ONLY. THIS GRANT ROUND HAS BEEN PROCESSED.
First 2018 Grant Opportunity — $25k grant available, expressions of interest due June 30, 2018
First 2018 Grant Opportunity — $25k grant available, expressions of interest due June 30, 2018
Intensive Investigation of Multi-Hit Families Affected by Autism and Related Neurodevelopmental Disorders
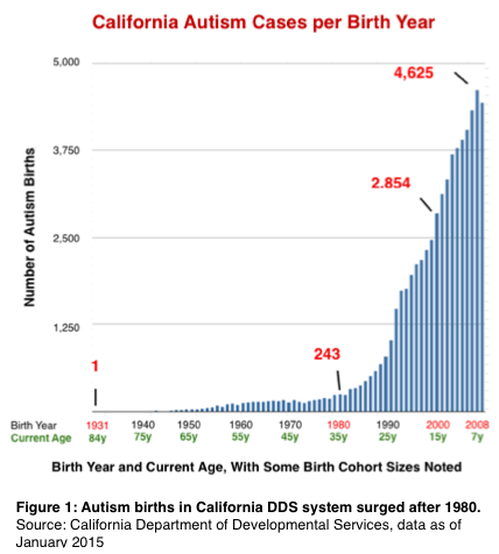
Few findings have helped explain the unprecedented surge in autism and related neurodevelopmental impairment that began with births in the 1980s. Given the high recurrence rate of autism among siblings and to a lesser extent, half-siblings in families with no prior history of the disorder, concern should be directed to potential exogenous sources of such serious heritable pathologies.
This RFP is intended to foster intensive investigation into exposure histories and characteristics of “multi-hit families” with three or more children (including half-siblings) affected by autism and related disorders. The related disorders include but are not limited to ADHD, Aspergers, learning disabilities and intellectual disability, social-communication impairments, speech and language impairments, ODD, IED, mood disorders, anxiety, depression, and schizophrenia spectrum and psychotic disorders.
A primary aim of this investigation would be to intensively interrogate multi-hit family histories — including, where possible, personal interaction with those families — to ascertain the possibility of acute exposures that may have impaired the molecular integrity of parental gametes, and in particular the gametes’ vulnerable precursor cells. Other approaches and topics may also also be included.
Given the limited size of the grant, it is directed at efforts to (1) identify or develop a multi-hit cohort, and (2) engage in at least some hypothesis hunting efforts by examining previously unconsidered factors in these strongly affected families. Follow-up grants to expand on initial findings may be available.
Informal expressions of interest should include the following:
(1) A title for the project and basic information about the investigators.
(2) Description of how a cohort of multi-hit families will be identified and/or recruited, either through community recruitment and/or drawing from a subset of existing cohorts. Families must have at least one offspring with autism and at least two siblings (including half-sibings) with autism or any related pathologies, such as those listed above. The rare families known to be affected by identified autism-related mutations present in both parental and affected offspring should be excluded from the cohort. Offspring with known de novo mutations may be included.
(3) Description of the proposed project, which may include any combination of:
Again, it is understood that the funds are only sufficient to support a pilot phase investigation.
(4) Description of how the funds (up to $25k) would be used. Please reference possible leveraging of data and data gathering from existing studies. If the work is to be done in phases, please describe those phases and the expected budgets for each.
How to apply
Informal expressions of interest are due no later than June 30, 2018. The document should be no longer than three pages and can be in formatted as text in an email rather than an attachment. Please email expressions of interest (questions prior to that are welcome) to [email protected].
Authors of submissions meeting review criteria will be invited to submit more detailed applications in the following months. We reserve the right to make changes to this RFP at any time for any reason, and changes will be posted at germlineexposures.org/grants.
Date: February 12, 2018. Other grant opportunities will be announced soon.
The Escher Fund for Autism is a donor-advised fund at Schwab Charitable.
This RFP is intended to foster intensive investigation into exposure histories and characteristics of “multi-hit families” with three or more children (including half-siblings) affected by autism and related disorders. The related disorders include but are not limited to ADHD, Aspergers, learning disabilities and intellectual disability, social-communication impairments, speech and language impairments, ODD, IED, mood disorders, anxiety, depression, and schizophrenia spectrum and psychotic disorders.
A primary aim of this investigation would be to intensively interrogate multi-hit family histories — including, where possible, personal interaction with those families — to ascertain the possibility of acute exposures that may have impaired the molecular integrity of parental gametes, and in particular the gametes’ vulnerable precursor cells. Other approaches and topics may also also be included.
Given the limited size of the grant, it is directed at efforts to (1) identify or develop a multi-hit cohort, and (2) engage in at least some hypothesis hunting efforts by examining previously unconsidered factors in these strongly affected families. Follow-up grants to expand on initial findings may be available.
Informal expressions of interest should include the following:
(1) A title for the project and basic information about the investigators.
(2) Description of how a cohort of multi-hit families will be identified and/or recruited, either through community recruitment and/or drawing from a subset of existing cohorts. Families must have at least one offspring with autism and at least two siblings (including half-sibings) with autism or any related pathologies, such as those listed above. The rare families known to be affected by identified autism-related mutations present in both parental and affected offspring should be excluded from the cohort. Offspring with known de novo mutations may be included.
(3) Description of the proposed project, which may include any combination of:
- Interviews and questionnaires. These would investigate the possibility of acute exposures to substances with demonstrated adverse mutagenic, epimutagenic and/or other germline effects (eg, tobacco and certain drugs, including synthetic steroid hormones and general anesthetic agents, medical radiation) during critical windows of parental germline development, most notably parental prenatal and neonatal periods. Direct interviews with family members, particularly parents and grandparents of affected offspring, are strongly recommended. [Think detective work, Autism CSI. When elevated lead levels were detected in my son's blood at the age of 12, a team of five county workers arrived at my door, sleeves rolled up and clipboards in hand, to investigate every possible avenue of exposure (there was no mystery, he had taken to impulsively peeling and eating paint out in the community). Yet, when two of my children as toddlers displayed catastrophically abnormal neurodevelopment, labelled autism—a vastly more serious and socially costly condition than the elevated lead level — not a single authority ever asked a single question about our family history of exposure in an attempt to understand what could have caused such unprecedented, devastating mental impairment. Not a single person arrived at our door. This stark contrast, and what I see as a possible untapped goldmine of information within families, inspired this RFP. —JE]
- Record review. In some cohorts, relevant information may be available in parents’ early life medical or other records. For example, a parent’s prematurity or birth complications may have some relationship to adverse endogenous or exogenous exposures. Similarly, if a parent was born to an older mother or a mother with a chronic condition like T1 diabetes or mental illness, or was born of any other pregnancy that was considered at-risk, that may serve as a proxy for treatment with certain drugs. Perhaps there are records of a parent’s gestational exposure to non-medical exposures such as smoking. Although a gumshoe detective approach is preferred, a big data approach to probing a variety of parental variables in association with abnormal neurodevelopmental outcomes in offspring might be interesting and fundable.
- Biological testing. This could include genomic testing and/or testing of paternal sperm for germline-level defects. Somatic exposures of the affected offspring may also be probed, including, for example, testing for maternal autoantibodies implicated in autism risk in multiplex families.
Again, it is understood that the funds are only sufficient to support a pilot phase investigation.
(4) Description of how the funds (up to $25k) would be used. Please reference possible leveraging of data and data gathering from existing studies. If the work is to be done in phases, please describe those phases and the expected budgets for each.
How to apply
Informal expressions of interest are due no later than June 30, 2018. The document should be no longer than three pages and can be in formatted as text in an email rather than an attachment. Please email expressions of interest (questions prior to that are welcome) to [email protected].
Authors of submissions meeting review criteria will be invited to submit more detailed applications in the following months. We reserve the right to make changes to this RFP at any time for any reason, and changes will be posted at germlineexposures.org/grants.
Date: February 12, 2018. Other grant opportunities will be announced soon.
The Escher Fund for Autism is a donor-advised fund at Schwab Charitable.
ARCHIVE ONLY. THESE GRANT ROUNDS HAVE BEEN PROCESSED.
2017 Grant Opportunity
Germline-Borne Etiology of Autism and Dysregulated Neurodevelopment:
The Question of Early Gamete Exposure to General Anesthesia
$25,000 Grant; Letters of interest due July 31, 2017
Background
The Escher Fund for Autism is devoted to piloting novel research into the germline-based etiology of autism spectrum disorders and related pathologies of early neurodevelopment. We are pleased to announce a new grant opportunity.
The Fund understands autism as a highly heritable condition (among siblings), with surprising rates of de novo and heterogenous mutations, inviting the question of how germline disruptions may have been induced within parental gametes. As DNA represents only one piece of molecularly controlled heritability—our work takes a "whole gamete" view of potential autism causation, including past exposures that may have upset regulatory, epigenomic, and cytoplasmic elements.
Generally speaking, our grants involve investigation of three variables:
The Fund specializes in funding small pilot projects on which later, and larger, grants could be based. Please learn more about the Fund and the germline disruption hypothesis of autism at germlineexposures.org.
The present RFP:
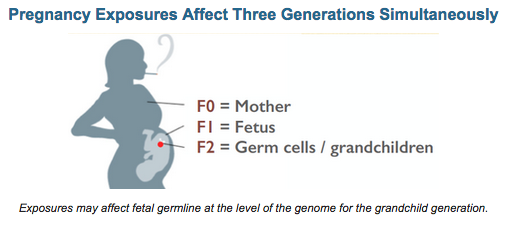
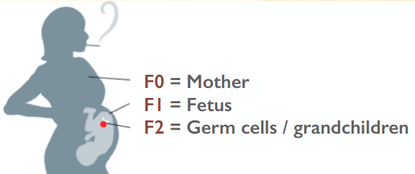
The present RFP concerns the hypothesis that early germline exposure to general anesthetic agents can raise the risk for heritable, gamete-borne, neurotoxicity in the following generation, via any number of potential molecular mechanisms, including those discussed above. The window of exposure would be while the F1 autism parent of the F2 autism child was in utero of the F0 grandmother given GA for surgery, and/or when the F1 autism parent was an infant undergoing a surgical procedure.
The Question of Early Gamete Exposure to General Anesthesia
$25,000 Grant; Letters of interest due July 31, 2017
Background
The Escher Fund for Autism is devoted to piloting novel research into the germline-based etiology of autism spectrum disorders and related pathologies of early neurodevelopment. We are pleased to announce a new grant opportunity.
The Fund understands autism as a highly heritable condition (among siblings), with surprising rates of de novo and heterogenous mutations, inviting the question of how germline disruptions may have been induced within parental gametes. As DNA represents only one piece of molecularly controlled heritability—our work takes a "whole gamete" view of potential autism causation, including past exposures that may have upset regulatory, epigenomic, and cytoplasmic elements.
Generally speaking, our grants involve investigation of three variables:
- High-dose exposures to substances with demonstrated adverse genomic, epigenomic and/or other germline effects (eg, tobacco and certain drugs, including synthetic hormones);
- During critical windows of germline development and reprogramming (mainly but not exclusively fetal germline, or primordial germ cells); and
- Adverse neurodevelopmental outcomes in the progeny of the toxicant-exposed germ cells, including but not limited to autism spectrum disorders.
The Fund specializes in funding small pilot projects on which later, and larger, grants could be based. Please learn more about the Fund and the germline disruption hypothesis of autism at germlineexposures.org.
The present RFP:
The present RFP concerns the hypothesis that early germline exposure to general anesthetic agents can raise the risk for heritable, gamete-borne, neurotoxicity in the following generation, via any number of potential molecular mechanisms, including those discussed above. The window of exposure would be while the F1 autism parent of the F2 autism child was in utero of the F0 grandmother given GA for surgery, and/or when the F1 autism parent was an infant undergoing a surgical procedure.
Why general anesthesia?
The reasons for focusing on general anesthesia (GA) in this grant round are as follows:
- GA agents are known to have epigenomically adverse effects. It has been shown that general anesthetics can cause substantial changes in gene and protein expression.
- The one animal study to investigate neurological toxicity in the F2 generation following F1 fetal exposure to a common GA agent found such an association. See the study here. ("[T]he learning retardation noted in the second generation born to mice exposed to halothane in utero, suggests that the anesthetic agent may have caused a genetic aberration.")
- Through a series of interviews with autism families, the Escher Fund has detected what appears to be an increased risk for multiplex idiopathic autism and other neurodevelopmental abnormalities in F2s where either (1) F0 grandmothers were subjected to GA while pregnant, for example, for an emergency appendectomy, (thereby exposing the F1 in utero) or (2) where an F1 parent had GA in early infancy for surgery. The fact that this exposure seems to be connected to having more than one F2 child with autism possibly points to GA as a a potent germline toxicant.
Proposals for this grant are limited to human cohorts. Possible outcomes to evaluate in the F2 may include:
- Phenotype: Abnormal behavior or neurodevelopment of F2 (eg, autism spectrum disorder, ADHD, anxiety, learning disabilities, mental illness, sensory processing disorders, other pathologies)
- Genotype/epigenotype: Abnormal molecular signatures in F2 proxy tissue and/or F1 sperm of exposed F1 fathers, including mutation and/or abnormal DNA methylation patterns.
Process for Submission of Proposals:
Informal expressions of interest are due July 31, 2017. The document should be less than two pages and can be in an email rather than in an attachment. It should include: (1) Names of the researchers, (2) a title, (3) a statement of the research question, (4) population studied, (5) outcome of interest (phenotype and/or biological outcome) and (5) a description of how this funding may leverage an existing study to study the F2 autism generation.
Please email your expression of interest to [email protected] by 5pm PST July 31, 2017. Those meeting review criteria will be invited to submit a more detailed application in the following months.
Escher Fund for Autism reserves the right to make changes to this RFP at any time for any reason, and changes will be posted here.
More Information from Escher Fund for Autism:
• Expert Q&As
• Germline in the News
• Blog
Questions are welcome and should be addressed to Jill Escher, [email protected]
Thank you to Dr. Robert Block, University of Iowa Department of Anesthesia.
2016 Grant Awards
[From our Fall 2016 newsletter]
New Grants: Couldn't Choose Just One...
Thank you to all the researchers who submitted proposals for the recent $25k RFP, Exogenously Induced De Novo Germline Errors (Genetic or Epigenetic) in the Etiology of Autism Spectrum Disorders. There were so many fantastic submissions it was impossible to choose just one. With apologies to our pocketbook, here are the award recipients:
• Marc Weisskopf, PhD, Harvard University
In the Nurses' Health Study II, a project to examine neurodevelopmental and behavioral outcomes in grandchildren of pregnant women given the toxic synthetic estrogen drug DES (diethylstilbestrol) or who smoked in pregnancy.
• Jonathan Sebat, PhD, University of California, San Diego
A project to ascertain via questionnaire the exposure of grandmaternal smoking in a cohort of families with children with autism.
• Jean Golding, PhD, Marcus Pembrey, PhD, and Alan Emond, MD, University of Bristol, UK
A project to continue work from prior Escher Fund grant examining associations between grandmaternal smoking and adverse neurodevelopmental outcomes in grandchildren in the Avon Longitudinal Study of Parents and Children.
Congratulations to all.
New Grants: Couldn't Choose Just One...
Thank you to all the researchers who submitted proposals for the recent $25k RFP, Exogenously Induced De Novo Germline Errors (Genetic or Epigenetic) in the Etiology of Autism Spectrum Disorders. There were so many fantastic submissions it was impossible to choose just one. With apologies to our pocketbook, here are the award recipients:
• Marc Weisskopf, PhD, Harvard University
In the Nurses' Health Study II, a project to examine neurodevelopmental and behavioral outcomes in grandchildren of pregnant women given the toxic synthetic estrogen drug DES (diethylstilbestrol) or who smoked in pregnancy.
• Jonathan Sebat, PhD, University of California, San Diego
A project to ascertain via questionnaire the exposure of grandmaternal smoking in a cohort of families with children with autism.
• Jean Golding, PhD, Marcus Pembrey, PhD, and Alan Emond, MD, University of Bristol, UK
A project to continue work from prior Escher Fund grant examining associations between grandmaternal smoking and adverse neurodevelopmental outcomes in grandchildren in the Avon Longitudinal Study of Parents and Children.
Congratulations to all.
2016 Grant Opportunities
Exogenously Induced De Novo Germline Errors (Genetic or Epigenetic) in the Etiology of Autism Spectrum Disorders
One $25,000 Grant; Three $5,000 Mini-Grants
DEADLINE HAS PASSED. THIS INFORMATION IS ARCHIVAL.
The Escher Fund for Autism is devoted to improving research into the germline-based etiology of autism spectrum disorders, with particular attention to the possibility of de novo molecular errors induced by exogenous agents, including grand-maternal pregnancy smoking and pharmaceuticals. Grants are intended to explore the hypothesis that a subset of ASD cases may result from unanticipated disruptions to vulnerable fetal germline programming caused by these or other evolutionarily novel and acute exposures common during the second half of the 20th century. Most studies will involve F0 pregnancy exposures of the 1950s-70s, and outcomes in F2s born 1980s-today.
Pregnancy Exposures Affect Three Generations Simultaneously
Exposures may affect fetal germline at the level of the genome for the grandchild generation.
These grants are not concerned with "generation-skipping" or "transgenerational" effects, but rather only with developmental consequences of direct exposures to early germ cells, which is a period of particularly acute vulnerability owing to dynamic molecular remodeling of germline.
The Fund specializes in funding small pilot projects on which later, and larger, grants could be
based. Please learn more about the Fund and the germline disruption hypothethesis of autism at germlineexposures.org.
Proposals must address at least one the following:
1. Early F1 Parental Exposure to Cigarette Smoke. In a human cohort or animal model, examination of F2 neurodevelopmental / behavioral and/or genomic/epigenomic outcomes where F0 pregnancies involved sustained and heavy cigarette smoking by F0 grandmothers. Alternatively or additively, the same outcomes involving F1 paternal cigarette smoking (less favored than previous model). It is established that cigarette smoke has both mutagenic and epimutagenic effects, and can adversely affect germ cell quality. The Escher Fund has detected what appears to be an increased risk for idiopathic autism and other neurodevelopmental abnormalities in F2s where F0 grandmothers were heavy smokers. This may be due to increased risk for de novo mutation and/or epimutation in F1 germline from which the F2s were derived.
2. Early F1 Parental Exposure to General Anesthesia. In a human cohort or animal model, examination of F2 neurodevelopmental / behavioral outcomes and/or genomic/epigenomic outcomes where F0 pregnancies or F1 neonates (maternal or paternal) had undergone general anesthesia (GA) for a surgical or medical procedure. It has been shown that general anesthetics can cause substantial changes in gene and protein expression. The Escher Fund has detected what appears to be an increased risk for multiplex idiopathic autism and other neurodevelopmental abnormalities in F2s where either (1) F0 grandmothers were subjected to GA while pregnant, for example, for an emergency appendectomy, and (2) where an F1 parent had GA in early infancy for surgery. Poor F2 neurodevelopmental outcomes may be due to increased risk for de novo mutation and/or epimutation in germline from which the F2s were derived. The fact that this exposure seems to be connected to having more than one F2 child with autism possibly points to GA as a a potent germline toxicant.
3. Early F1 Parental Exposure to Synthetic Steroid Hormone Drugs. In a human cohort or animal model, examination of F2 neurodevelopmental / behavioral outcomes and/or genomic/epigenomic outcomes where F0 pregnancies involved sustained and heavy exposures to synthetic steroid hormone drugs, such as DES (diethylstilbestrol) and/or progestin drugs that were popular (yet ineffective) in "anti-miscarriage" practice in the 1950s-80s. These drugs were widely used in pregnancies considered to be "at risk," for example in women who had prior miscarriages, were of older age, or who were carrying twins. It has been shown that synthetic steroids and endocrine disrupting chemicals that mimic but do not faithfully duplicate hormone action can have adverse epigenomic effects that persist in later generations, via perturbation of germline.
Please note: Kinsey File Project: The Escher Fund is interested in partnering with a researcher willing to design a study to ascertain F2 outcomes of the 71 Los Angeles F1 offspring prenatally exposed to synthetic steroid hormone drugs, and evaluated in this 1977 study by Dr. June Reinisch. (Jill Escher, who has two children with idiopathic autism, was among those study subjects). The original files from the 1977 study still exist, with full records of the pharmaceuticals to which the F1s were exposed in utero, and are currently stored at the Kinsey Institute in Indiana. There may also be files from a similar cohort based in New Jersey. Further details available by emailing [email protected].
The Fund specializes in funding small pilot projects on which later, and larger, grants could be
based. Please learn more about the Fund and the germline disruption hypothethesis of autism at germlineexposures.org.
Proposals must address at least one the following:
1. Early F1 Parental Exposure to Cigarette Smoke. In a human cohort or animal model, examination of F2 neurodevelopmental / behavioral and/or genomic/epigenomic outcomes where F0 pregnancies involved sustained and heavy cigarette smoking by F0 grandmothers. Alternatively or additively, the same outcomes involving F1 paternal cigarette smoking (less favored than previous model). It is established that cigarette smoke has both mutagenic and epimutagenic effects, and can adversely affect germ cell quality. The Escher Fund has detected what appears to be an increased risk for idiopathic autism and other neurodevelopmental abnormalities in F2s where F0 grandmothers were heavy smokers. This may be due to increased risk for de novo mutation and/or epimutation in F1 germline from which the F2s were derived.
2. Early F1 Parental Exposure to General Anesthesia. In a human cohort or animal model, examination of F2 neurodevelopmental / behavioral outcomes and/or genomic/epigenomic outcomes where F0 pregnancies or F1 neonates (maternal or paternal) had undergone general anesthesia (GA) for a surgical or medical procedure. It has been shown that general anesthetics can cause substantial changes in gene and protein expression. The Escher Fund has detected what appears to be an increased risk for multiplex idiopathic autism and other neurodevelopmental abnormalities in F2s where either (1) F0 grandmothers were subjected to GA while pregnant, for example, for an emergency appendectomy, and (2) where an F1 parent had GA in early infancy for surgery. Poor F2 neurodevelopmental outcomes may be due to increased risk for de novo mutation and/or epimutation in germline from which the F2s were derived. The fact that this exposure seems to be connected to having more than one F2 child with autism possibly points to GA as a a potent germline toxicant.
3. Early F1 Parental Exposure to Synthetic Steroid Hormone Drugs. In a human cohort or animal model, examination of F2 neurodevelopmental / behavioral outcomes and/or genomic/epigenomic outcomes where F0 pregnancies involved sustained and heavy exposures to synthetic steroid hormone drugs, such as DES (diethylstilbestrol) and/or progestin drugs that were popular (yet ineffective) in "anti-miscarriage" practice in the 1950s-80s. These drugs were widely used in pregnancies considered to be "at risk," for example in women who had prior miscarriages, were of older age, or who were carrying twins. It has been shown that synthetic steroids and endocrine disrupting chemicals that mimic but do not faithfully duplicate hormone action can have adverse epigenomic effects that persist in later generations, via perturbation of germline.
Please note: Kinsey File Project: The Escher Fund is interested in partnering with a researcher willing to design a study to ascertain F2 outcomes of the 71 Los Angeles F1 offspring prenatally exposed to synthetic steroid hormone drugs, and evaluated in this 1977 study by Dr. June Reinisch. (Jill Escher, who has two children with idiopathic autism, was among those study subjects). The original files from the 1977 study still exist, with full records of the pharmaceuticals to which the F1s were exposed in utero, and are currently stored at the Kinsey Institute in Indiana. There may also be files from a similar cohort based in New Jersey. Further details available by emailing [email protected].
Possible outcomes to evaluate in the F2 include:
Phenotype: Abnormal behavior or neurodevelopment of F2 (eg, autism spectrum disorder, ADHD, anxiety, learning disabilities, mental illness, sensory processing disorders, other pathologies)
Genotype/epigenotype: Abnormal molecular signatures in F2 proxy tissue and/or F1 sperm, including mutation and/or abnormal DNA methylation patterns.
Review criteria for $25k Grant include:
• Use of existing resources such as extant autism cohorts to extend the span of inquiry of autism parent participants to include exogenous agents affecting early paternal or maternal germline.
• Biological plausibility of hypothesis being tested (eg, the more genotoxic and acute the exposures the better, and in critically vulnerable periods of germline synthesis).
• Use of existing autism genomic data to search for clues or biomarkers of precipitating F0 or F1 exposures.
• Preference for projects done in phases, with grant monies disbursed in phases.
• Preference for Kinsey File project.
Process for Submission of Proposals for $25k grant:
Informal expressions of interest due: July 31, 2016
The proposal process is informal. Please email a rough outline of your proposal as a PDF or text to [email protected] by July 31, 2016. Those rough proposals meeting review criteria will be invited to submit a more detailed two-page application.
DEADLINE HAS PASSED.
Award notifications will be made no later than end of the calendar year 2016.
No portion of these funds shall be used to cover indirect university costs.
Escher Fund for Autism reserves the right to return any proposal that is not responsive to its research goals or exceeds its funding limits or available resources, at any time during the application and review process. EFA reserves the right to make changes to this RFA at any time for any reason, and changes will be posted under “Grants” at germlineexposures.org.
Process for Submission of Proposals for Mini-grants:
In 2016, three mini-grants in the $250-$5,000 range are available on a rolling basis to support meetings, papers, research, or other work related to investigations of the induced germline disruption hypothesis of autism. Simply email us with your inquiry, the process is informal.
More Information:
The Escher Fund for Autism supports projects that investigate the role of de novo germline perturbation in the etiology of autism and related disorders. Visit GermlineExposures.org for:
• Expert Q&As
• Germline in the News
• Blog
• New! Slides from 2016 "Out of the Past: Old Exposures, Heritable Effects, and Emerging Concepts for Autism Research"
Questions should be addressed to Jill Escher, [email protected]
Phenotype: Abnormal behavior or neurodevelopment of F2 (eg, autism spectrum disorder, ADHD, anxiety, learning disabilities, mental illness, sensory processing disorders, other pathologies)
Genotype/epigenotype: Abnormal molecular signatures in F2 proxy tissue and/or F1 sperm, including mutation and/or abnormal DNA methylation patterns.
Review criteria for $25k Grant include:
• Use of existing resources such as extant autism cohorts to extend the span of inquiry of autism parent participants to include exogenous agents affecting early paternal or maternal germline.
• Biological plausibility of hypothesis being tested (eg, the more genotoxic and acute the exposures the better, and in critically vulnerable periods of germline synthesis).
• Use of existing autism genomic data to search for clues or biomarkers of precipitating F0 or F1 exposures.
• Preference for projects done in phases, with grant monies disbursed in phases.
• Preference for Kinsey File project.
Process for Submission of Proposals for $25k grant:
Informal expressions of interest due: July 31, 2016
The proposal process is informal. Please email a rough outline of your proposal as a PDF or text to [email protected] by July 31, 2016. Those rough proposals meeting review criteria will be invited to submit a more detailed two-page application.
DEADLINE HAS PASSED.
Award notifications will be made no later than end of the calendar year 2016.
No portion of these funds shall be used to cover indirect university costs.
Escher Fund for Autism reserves the right to return any proposal that is not responsive to its research goals or exceeds its funding limits or available resources, at any time during the application and review process. EFA reserves the right to make changes to this RFA at any time for any reason, and changes will be posted under “Grants” at germlineexposures.org.
Process for Submission of Proposals for Mini-grants:
In 2016, three mini-grants in the $250-$5,000 range are available on a rolling basis to support meetings, papers, research, or other work related to investigations of the induced germline disruption hypothesis of autism. Simply email us with your inquiry, the process is informal.
More Information:
The Escher Fund for Autism supports projects that investigate the role of de novo germline perturbation in the etiology of autism and related disorders. Visit GermlineExposures.org for:
• Expert Q&As
• Germline in the News
• Blog
• New! Slides from 2016 "Out of the Past: Old Exposures, Heritable Effects, and Emerging Concepts for Autism Research"
Questions should be addressed to Jill Escher, [email protected]
ARCHIVE ONLY
2015 Grant Opportunities
|
We are pleased to announce the winner of the June 30, 3015 $25k grant. Congratulations to Carlos Guerrero-Bosagna of Linköping University, Sweden, for his proposal, "Germ-line Epigenetic Marks of Ancestral Exposures Correlated to Neurodevelopmental Disorders." The co-applicants were Dr. Anita Ost and Dr. Heriberto Martinez-Rodriguez, IKE, Faculty of Health Sciences. Using novel techniques, combined with Sweden's excellent medical records, the team aims to identify in human sperm samples epigenetic marks that could indicate past exposure to circumstances correlated with the incidence of neurodevelopmental disorders. Congratulations!
$25k RFP: Early Germline Exposures in the Histories of Autism Multiplex Parents
Applications due June 30, 2015 This RFP is intended to support research concerning the exposure histories of people (“F1”) born in the 1950s or thereafter and who have more than one biological child (”F2”) with idiopathic autism spectrum disorder or at least one child with ASD and at least one other child with a related idiopathic neurodevelopmental disorder such as ADHD, intellectual disability, or mental illness. We hypothesize that a subset of these pathologies arise from induced epigenomic and/or genomic disruptions of parental germline, either of the father’s sperm, or the mother’s eggs. Of interest to us for the purposes of this RFP is not the time of conception, but instead the vulnerable phase of fetal germline synthesis, which occurs approximately 20-40 years prior to conception, during the parents’ (F1) own fetal development. This dynamic phase involves specification and proliferation of germ cells, epigenetic reprogramming, and the laying of genomic imprints, among other phenomena. And in the 1950s, 60s, and 70s, these phenomena occurred in the context of many novel and toxic gestational exposures. Birth cohort data from California’s Department of Developmental Services (see Figures 1 and 3) indicate that autism births began a quasi exponential ascent starting in the early 1980s. This growth in DDS-eligible autism — a subset of autism involving more severe mental disability and functional limitations, and excluding the “high functioning” segment — has been repeatedly determined not attributable to diagnostic shift or broadening of eligibility criteria.1 California DDS autism numbered about 3,000 in the mid-1980s. Today the autism caseload exceeds 75,000.
According to the germline disruption hypothesis, a portion of this surge may result from developmental dysregulation resulting from molecular effects of old and largely forgotten exposures to the F1 parental germline. For developmental pathology in F2 offspring to have occurred in births in the 1980s and thereafter, one should consider germline errors occurring during the most vulnerable phase of F1 germline synthesis, that is during F1’s own fetal development, in the 1950s and thereafter. Assuming induced germline errors may be playing a role in the autism surge, the next logical question is what exposures increasingly affected the uterine environment in the 1950s, 60s, 70s, exposures that perhaps had been nonexistent or less common in prior decades, centuries, or even millennia? The answers could lie in many evolutionarily novel stressors, some of which are noted in Figure 2. For example, maternal cigarette smoking became ubiquitous and peaked in the 1960s. Moreover, a wide variety of novel substances were invented, marketed and used as pregnancy drugs, including synthetic “anti-miscarriage” hormones, anti-nausea drugs, painkillers, psychoactive drugs such as antidepressants and anti-anxiety medications, sleeping aids and sedatives, anesthesia, and more. Many of those drugs had powerful endocrine-disrupting or otherwise toxic effects. Medical radiation was applied to many pregnancies. Recreational drug use also surged in the 1960s.
We hypothesize that many of these abnormal post-war in utero exposures to the parent generation (F1) could have acted as subtle fetal germline teratogens, inducing mutagenesis, epimutagenesis or both, resulting in dysregulation of development of the subsequent generation (F2). We further hypothesize that fetal germline exposures can result in heritability of autism or related neurodevelopmental disorders due to the likelihood of multiple gametes being affected by the same transient exposure (thus the focus here on multiplex families). It would also result in heterogeneity because, in part, different gametes would be challenged in different ways by an exposure. Gender differences may manifest owing to epigenomic mechanisms that result in differential phenotypes according to gender, such as imprinting and X-chromosome inactivation. It would also result in demographic differences, since many of the exposures (for example, once-popular prophylactic synthetic hormone treatments) tended to be available to women of higher SES and located in metropolitan areas. |
No Deadline
Mini-grants in the $250-$5,000 range are available on a rolling basis to support meetings, papers, research, or other work related to investigations of the induced germline disruption hypothesis of autism. Simply email us with your inquiry. The Escher Fund for Autism supports projects that investigate the role of de novo germline perturbation in the etiology of autism and related disorders. Visit GermlineExposures.org for:• Expert Q&As • Germline epigenetics in the news• Germline development and reprogramming backgrounder• Blog Email: jill.escher@gmail.com |
|
fn1: 1 See, for example, Autistic Spectrum Disorders, Changes in the California Caseload An Update: June 1987 – June 2007; and The Epidemiology of Autism in California (2002), noting “The observed increase in autism cases cannot be explained by a loosening in the criteria used to make the diagnosis,” and stating, “Has the increase in cases of autism been created artificially by having ‘missed’ the diagnosis in the past, and instead reporting autistic children as ‘mentally retarded?’ This explanation was not supported by our data.” Moreover, there is no signal or evidence that California’s singularly robust developmental services system has overlooked tens of thousands of mentally and developmentally disabled adults born prior to the 1980s (now over the age of 35) with the striking autism symptomology.
To apply: No later than June 30, 2015, applicants should email the following information to [email protected]: (1) Project description: Please provide an overview, in 1000 words or less, of your proposed project probing the possibility of germline disruption in the histories of multiplex autism families. Some examples of relevant projects (these are only examples): • Case studies and assays of ASD multiplex families. Genetic and epigenetic analysis of multiplex offspring (F2) of parents (F1) exposed in utero to stressors such as pharmaceutical drugs or smoking. • Surveys of multiplex families. In a cohort of F2 multiplex families, use of a survey to ascertain potential germline exposures of the F1 parents. • Epidemiology: Given a cohort of F1 parents with documented prenatal exposures, is there an elevated risk of multiple F2 offspring with developmental pathologies? • Human semen analysis: Analysis of human F1 semen where there is more than one F2 with ASD or related neurodevelopmental disorders, with an attempt to ascertain an exposure biomarker. • Meetings, symposia, or publications: Sessions at scientific meetings, conference panels or symposia, articles or publications about this topic. Please note: Abnormal exposures of greatest interest include pharmaceutical, recreational drug, or medical treatments. Synthetic steroid hormone drugs (progestins, corticosteroids, fake estrogens) there were used prolifically in obstetric practice (typically in old “anti-miscarriage” protocols) and in current IVF and/or anti-preterm birth practices (eg, Makena, the progestin 17-OHPC) are of greatest, though not exclusive, interest. Abnormal molecular outcomes of interest may include but are not limited to: abnormal methylation of male or female germline, or other epigenetic marks, de novo mutation in male or female germline, or any other genetic variation not descended from the parental DNA. Multiplex for abnormal neurodevelopmental outcomes. Families studied must include at least one F2 child with autism, and a sibling with autism or Aspergers, PDD-NOS, ADHD/ ADD, sensory processing disorders, learning disabilities, social development disorders, communication disorders, behavioral/conduct disorders, cognitive deficits, or mental disability or illness. Studies investigating exposure histories of families with three of more biological siblings with idiopathic neurodevelopmental abnormalities would be of great interest. Early gametogenesis only: This grant is limited to projects investigating the critical window of germline development and epigenetic reprogramming during the period of F1 embryogenesis and fetal development, beginning with the specification of the primordial germ cells within the F1 (parent generation). In other words, when the grandmother (F0)was gestating the future autism parent (F1) and its germline (F2), did the grandmother take drugs or pharmaceuticals or smoke? (2) Collaborators. CVs can be emailed or linked separately. Interdisciplinary work, for example, involving a toxicologist, geneticist or epigeneticist, developmental or reproductive biologist, epidemiologist, and/or medical historian, is preferred. (3) Budget: Line item description of how grant money would be used. The applicant must stipulate that max 5% of grant funds will support indirect costs. (4) Timeline: Projects must be completed within a year of the grant. (5) Amount of request: $25,000 is the maximum, but requests for smaller amounts will also be considered and may be given priority. This grant may be used to augment or leverage other awards or projects. We realize this amount may only be enough to fund a small pilot or “proof of principle” project. Depending on grantee progress, further support may be available. (6) Tax status of requestor: Grants can be given through 501c3 or otherwise qualifying nonprofit organizations or institutions only. Deadline to submit proposals to [email protected] is June 30, 2015. Investigators should feel free to email us with any questions or to informally discuss proposals prior to submission. We reserve the right to modify this RFP prior to June 1, 2015. [ARCHIVE] Request for Proposals: The Genetics of Autism
20th Century Maternal Smoking: Induced Fetal Germline Perturbations in the Etiology of Autism and Neurodevelopmental Disorders Application deadline: February 27, 2015; $25,000 grant available [We are pleased to announce the winner of the February 27, 3015 $25k grant. Congratulations to Dr. Jean Golding and her team at University of Bristol, UK, who will use the funds on their famed ALSPAC cohort. ]
Background:
The past three decades have seen a staggering increase in the number of children diagnosed with the neurodevelopmental disability of autism. In California, substantially disabling autism cases served by the Department of Developmental Services have soared more than 2,000% since the early 1980s, now surpassing 73,000 cases, prompting what many see as perhaps the greatest public health crisis in the state’s history, as virtually all these individuals have severe functional limitations and will require lifelong care. The skyrocketing rates of profound disability have baffled families, scientists and practitioners, because though autism appeared to be strongly heritable (among siblings, not parent-to-child), the idea of a “genetic epidemic” made little sense in light of the understanding that genes could not change so dramatically in the course of a single generation. It has come to light, however, that heritability includes de novo mutation of germline in addition to induced dysregulation of environmentally sensitive “epigenome.” Germ cells — eggs, sperm, and their precursors — contain countless millions of epigenetic marks that control how genes function, and these marks can be susceptible to perturbation in critical windows, including early gametogenesis, a time of dynamic and widespread epigenetic remodeling of chromatin. Disruption of germline epigenetics can cause permanent dysregulation of sensitive genes, including imprinted genes, leading to pathology in offspring. In short, it is now understood that maternal smoking — like other toxic exposures of pregnancy — can affect three generations simultaneously: the soma of the mother (F0), the soma of the fetus (F1), and the germ cells of the fetus, which become the F2, or grandchildren of the F0. If the F2 generation is perturbed, it would be at the root genetic or epigenetic level. Based on what we are learning from epigenetics and mutagenesis, reproductive and germ cell biology, and the science of endocrine disruption and toxicology, could widespread F0 maternal smoking of the 1950s, 60s, and 70s have caused unforeseen derangements to some fetal germline (the F2 within F1), giving rise to developmental or behavioral abnormality in a subset of the grandchild generation? While the sponsor is interested in a wide array of gestational toxicants, particularly the many powerful synthetic pregnancy drugs of the 20th century, this particular RFP is limited to investigations of intergenerational effects of maternal cigarette smoking, a pervasive, intensive toxic exposure and an established mutagen and epimutagen. To apply: (1) Project description: Please provide an overview, in 500 words or less, of your proposed project, including methods. Some examples of relevant projects: • Animal models: Given F0 cigarette smoking, what are F1 fetal germline and F2 behavioral outcomes? • Case studies of ASD families with parental prenatal smoking exposure: Genetic and epigenetic analysis of parents exposed in utero to maternal smoking, and same for their children. • Epidemiology: Given a cohort of pregnancies for which there are reliable prenatal medical records dating from circa 1960s, are F2 developmental outcomes different when the F0 grandmother had smoked, compared to controls? • Human semen analysis: Analysis of human F1 semen where there is known F0 pregnancy smoking, as compared to controls. Also in cases where F1 had fathered children with ASD. • Meetings, symposia, or publications: Sessions at scientific meetings or conference panels or symposia about this topic; articles or publications that help educate the science, regulatory, or lay community about fetal germline disruption. Please note: Abnormal molecular outcomes of interest may include but are not limited to: abnormal methylation of male or female germline, or other epigenetic marks, de novo mutation in male or female germline, or any other genetic variation not descended from the parental DNA. Abnormal phenotypic outcomes of interest may include but are not limited to: Autism spectrum disorders, Aspergers, PDD-NOS, ADHD/ ADD, Sensory processing disorders, Learning disabilities, Social development disorders, Communication disorders, Behavioral/conduct disorders, and Mental illness. Effects of second-hand smoke: in addition to direct germline exposure through maternal smoking, may also be considered. Chemical components of tobacco smoke: It is preferred that a project contemplate all chemical components of tobacco smoke in the second half of the 20th century, including DDT, other pesticides, cigarette additives, and radioactive materials. History of maternal smoking: it is preferred that a project include a brief overview of the history of maternal smoking in the United States in the 20th century. Critical windows: It is preferred that projects look at impacts on germline development and epigenetic reprogramming during the period of embryogenesis and fetal development, beginning with the specification of the primordial germ cells. However, projects investigating other critical windows such as periconception and/or spermatogenesis will also be considered. (2) Collaborators. CVs can be emailed or linked separately. Interdisciplinary work, for example, involving a toxicologist, geneticist or epigeneticist, developmental or reproductive biologist, and historian, is preferred. (3) Budget: Line item description of how grant money would be used. The applicant must stipulate that max 5% of grant funds will support indirect costs. (4) Timeline: Projects must be completed within a year of the grant. (5) Amount of request: $25,000 is the maximum, but requests for smaller amounts will also be considered and may be given priority. This grant may be used to augment or leverage other awards or projects. We realize this amount may only be enough to fund a small pilot or “proof of principle” project. (6) Tax status of requestor: Grants can be given through 501c3 or otherwise qualifying nonprofit organizations or institutions only. Depending on grantee progress, further support may be available. Deadline to submit proposals to [email protected] is February 27, 2015. Applicants are free to email comments or questions prior to submitting a proposal. Applicants seeking further information about germline development and vulnerabilities are encouraged to browse the expert interviews and germline development tabs on this website. |

About the Escher Fund for Autism
The Escher Fund for Autism (“Humans Start as Molecules”) sponsors the science education website germlineexposures.org and promotes and funds research into the hypothesis that adverse fetal exposures, along with exposures during other critical windows of germline synthesis, can induce molecular derangement of vulnerable fetal germline, giving rise to quasi-genetic pathologies in the next generation.
The founder is the mother of two children with nonverbal autism. The story of her discovery of in utero exposure to multiple synthetic hormone drugs is here. Though a forgotten history to most of today’s autism researchers, these powerful chemicals, along with many other drugs, were used prolifically during 1950s, 60s, and 70s to treat pregnancies considered “at risk” and many other real and perceived problems of pregnancy. This era also saw unprecedented rates of maternal smoking.
Family surveys conducted by the Escher Fund suggest that in utero exposure to drugs or smoking sustained by parents (when they were fetuses) and their nascent germline during the 1950s, 60s, and 70s may be a substantial causative factor in the surging rates of autism and related neurodevelopmental disorders in their offspring.
Past grantees of the Escher Fund for Autism, along with its sister fund, the Escher Family Fund, have included:
University of Bristol • Child Health and Development Survey • University of Copenhagen • UCSF • Harvard University • UCSD • Rockefeller University • Brown University • UCLA • Autism Speaks • Linkoping University • University of Chicago • Stanford University • Florida State University • North Carolina State University • Colorado State University • Columbia University • Keystone Symposia • Gordon Research Conferences • International Society for Autism Research • Environmental Mutagenesis & Genomics Society • Society of Toxicology • and more, along with dozens of autism service organizations.