Fetal Origins of Disease: Small Disruptions, Big Effects, with Carmen Marsit, PhD
"[A]ny changes, any kind of exposures, or any kind of stresses that happen during development ...
you can imagine that they can have lifelong impacts because now they're going to
change the way that those cells are able to function or behave."
|
Carmen Marsit, PhD
Associate Professor of Pharmacology, Toxicology, and of Epidemiology at Geisel School of Medicine at Dartmouth Marsit Epigenetics Laboratory website |
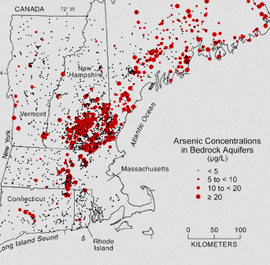
Carmen J. Marsit, PhD, is Associate Professor of Pharmacology, Toxicology and of Epidemiology at Geisel School of Medicine at Dartmouth, New Hampshire. Prior to Dartmouth, Dr. Marsit was an assistant professor in the Departments of Pathology and Laboratory Medicine and Community Health (Epidemiology) at Brown University. He holds a Bachelor of Science degree in Biochemistry from Lafayette College, and a PhD in the Biological Sciences in Public Health, focused on Cancer Biology, from Harvard University. Dr. Marsit was a post-doctoral fellow at the Harvard School of Public Health, where he gained training and interest in molecular epidemiology and epigenetics in cancer. During his postdoctoral training, he began his studies of DNA methylation alterations and variation in bladder cancer working with Dr. Karl Kelsey and Dr. Margaret Karagas at Dartmouth, considering not only their role in disease etiology but also their clinical importance as biomarkers of risk or prognosis. At Brown, he expanded his research program to examine the importance of epigenetic regulation in human development and the importance of the intrauterine environment on human health, focusing on mental health. He has successfully designed and executed the Rhode Island Child Health Study, a population-based birth cohort designed to examine the impact of placental epigenetic variation on early life neurobehavioral and other health outcomes. He continues to expand his work on the role of epigenetic mechanisms including DNA methylation and microRNA expression in the developmental origins of health and disease, as well as aiming to expand his work on bladder cancer epigenetics to the clinical setting. The Marsit Epigenetics Laboratory at the Geisel School of Medicine uses population-based studies and interdisciplinary approaches to better understand how the environment impacts human health and disease. Interviewed by Jill Escher, October 2014 Dr. Marsit, your work tackles such a broad field, how did you get into environmental epigenetics, and so many aspects of it? I was really interested in toxicology when I was thinking about going to grad school, and in trying to understand how the environment can impact health. Going into graduate school, through different labs, I ended up finding that I was really interested in working in populations and so I moved towards the epidemiology side. A lot of my early work was in cancer, trying to understand how environmental exposures can have epigenetic impacts in a tumor as it develops. People had started defining changes in DNA methylation and other epigenetic alterations in tumors, but nobody was really thinking about, why do they arrive, where do they come from? So we started looking at some of the environmental components. I started developing my own work and my own independent research and when I moved to Brown, I had this great opportunity to think about some of these same mechanisms but in the developmental context. Which, I think, absolutely, is where some of the most important mechanisms are occurring and where exposures could have some of the longest-term effects. We're talking about how exposures in utero are really changing the health of children and just learning more about that whole “developmental origins” area. So considering how environmental factors alter epigenetic mechanisms and how those alterations could be related to outcomes, at least in newborns and hopefully, as we move forward, looking at older children to try to better realize what some of these early changes could be doing. Could you take a little time to explain more about fetal origins of disease? Oh, absolutely. I think this is a really exciting area and something that we're still learning a lot about. It developed out of these large studies of individuals who were exposed to famine during WWII. In the Netherlands there was a blockade by the Nazis and food supplies were limited. So there were women who were pregnant at that time who had experienced famine during pregnancy. Subsequent to that these affected individuals were able to be followed into their adulthood and now they're quite elderly. Epidemiologists have been able to look at some of the effects that that famine exposure during gestation had on them. You could actually see very specific effects of famine depending on the timing of the exposure during gestation. This famine exposure related to things like mental health risks, schizophrenia, to diabetes and to obesity now when the children are older. That was some of the fundamental work, and this concept has been expanded now to look at other populations where studies have shown that children born with low birth weight also are at higher risks for obesity and metabolic diseases later in life, and other health outcomes. Also, there is literature in cancer related to developmental exposures. Women exposed to diethylstilbestrol (DES), not only the women exposed but also their children that were exposed in utero have been shown to be at risk for subsequent cancers later in life. I actually have a specific question related to DES later, so we'll get back to that. Fantastic. So there were epidemiologic studies linking these factors to outcomes, but now we're able to start looking at some of the mechanisms that might be involved. How does an exposure during nine months of pregnancy lead to changes throughout your entire life and potentially beyond your life into your children's lives? Trying to understand those mechanisms is really interesting. There's a way that the body is able to take a single genome so every cell in our body has the same genetic makeup, but every cell is different. Your skin is very different than your stomach and everything else. That is epigenetics. It's through those epigenetic mechanisms that we're able to elaborate on the genome to create all these specific cells. So we think that any changes, any kind of exposures, or any kind of stresses that happen during development when those mechanisms are getting set and setting up all of your cells, if those can be altered in any way, you can imagine that they can have lifelong impacts because now they're going to change the way that those cells are able to function or behave. Let's talk about those mechanisms during early development and why there are these windows of susceptibility in certain phases of the lifecycle. Why are certain phases of development are more plastic from a mechanistic point of view? Part of it is because it's set up to do that. You have to go from being a single cell to every cell of your body. So during that developmental period, cells are programmed to actually undergo these alterations and to start programming what they're going to become. A lot of that programming is done by certain cells excreting something to neighboring cells to tell them, "Okay, these cells closest to me are going to become the next types of neuron. The ones that get further away are going to end up becoming the spinal chord and so on moving down the line." A lot of the early programming is really about this distance. You have one cell excreting something that the further away the other cells are, the different patterns that they pick up -- You’re saying the cells are talking to each other? Absolutely, yes. How does that happen, through little RNAs? Sometimes, it may be through RNAs. It may be through secreted proteins or hormones that work on gradients and really set up the direction. The entire way that your head is defined compared to your legs and tail, if you're an animal, is by these gradients that get set up across cells. So they secrete these factors then the next cell picks it up. It gets less and less as it gets further away. That's how cells get told what they're going to become. Wow. So you can imagine that if there are specific exposures that are coming in that might look like some of these factors or might alter the way that some of these factors can actually do their job, then you can start altering the epigenetic mechanisms that really program the cell to do what it has to do. So what are some exposures that might mimic the signaling or disrupt the signaling? You can think of many different types of exposures. Endocrine disruptors or any kind of hormonal type exposures might cause problems. Even metals exposures, those might get into a cell and then be able to alter the way transcription happens. A lot of environmental exposures could be important. It may even be stressors in the environment. A mother experiencing extreme stress might herself be secreting stress hormones that then are able to pass onto the infant and change the way that infant is developing. The infant is going to see too much of those stress hormones when it really shouldn't be seeing them. I’m interested in multigenerational effects of maternal cigarette smoking. Do you know of factors in cigarette smoke that can be mutagenic or epimutagenic? There's probably very many. Cigarette smoke is so complicated and has so many chemicals. Anything from nicotine which we know can pass through the placenta, affecting the placenta and the developing infant. There's a number of heavy metals that are found in cigarette smoke that may be toxic. Cadmium is one that is very common in cigarette smoke that has neurotoxic effects; any many byproducts of a very dirty combustion can cause problems. Tobacco is laced with chemicals, things like benzo[a]pyrenes which are very reactive and have endocrine-disruptive type effects because they look sometimes like hormones. Some of these reaction products can look like hormones or mimic hormones. Those could certainly have effects. I think there are probably hundreds of chemicals in there that could be having effects — and I think piecing that out is a challenge because there are so many chemicals that are probably working with each other to make it even worse. I wanted to talk about DES because it's one of the few pharmaceuticals that's actually been studied with respect to epigenetic effects. Not only that, it's been studied at least a little bit as to second-generation effects. Of course I have a personal interest in DES. I was not a DES daughter, but was exposed to five other synthetic hormones that were used as anti-miscarriage drugs in the '60s. Why is it that hormone mimics have the long-term effects on tissues? DES mimics estrogen. Estrogen is such an important hormone during development and likely, estrogen plays a major role in setting down some of these epigenetic programs. We don't totally understand how that epigenetic landscape gets placed. Why do we see specific DNA methylation marks where they are? Likely it's because factors like estrogen can come in and bind to their receptors and then sit down on the DNA in certain places, likely directing some of those marks to be set where they need to become set in cells where they should be. So if you have a synthetic chemical like DES that can mimic estrogen but actually can be even stronger than the native hormone, you might be having this effect in places where you shouldn't necessarily see the effects, or just creating even more extreme effects and so then leading to these kinds of changes. What are we finding with the second generation effects? I know more on that area about the cancer outcomes. There have been studies that have identified risks for breast cancer, endometrial tumors or ovarian tumors in the second generation of women that were exposed to DES in utero. So DES would be affecting their germ cells. There's very strong evidence for those long term, multigenerational effects. And in males as well, right? That's right, yes. Yes, there are some new studies coming out that are looking also in males now for prostate cancer and other cancers, so, absolutely. Particularly in the reproductive tissues, I think. Again, it's because it is an extremely powerful estrogen-mimic. People have asked me about whether or not there have been any second generation studies with regard to behavioral outcomes because I'm interested in the neurodevelopmental results of germline perturbations. I do think it's an interesting area, and I think people are probably going to move in that direction. Well what's really interesting is so many of these mental health outcomes have sexually dimorphic effects that some affect men more often than women or girls more often than boys. So it would make sense in fact that something like DES could play a role in those effects. Because we know that somehow sex hormones are playing some role. So, it will be interesting to think about how that works. Speaking of F1 sexually dimorphic effects, when I was a kid I was enrolled in a study on the personality impacts of prenatal synthetic hormone exposures. People like me who were heavily exposed to synthetic hormones demonstrated behavior differences caused by our abnormal exposures. It's wacky how powerful these drugs are in early development. Regarding epidemiology, granted, a lot of things have changed in our environment, everything from ambient chemicals to personal care products to what we eat and what we breathe, but I tend to be a little bit more concerned about acute exposures like cigarette smoke and pharmaceuticals, antidepressants, the synthetic hormones, people are increasingly medicated for a variety of reasons. There's artificial reproductive technology which employs not only physical interference but a lot of chemical interference as well. Are there particular practices and exposures today that you think warrant more concern with respect to these long term epigenetic effects? I agree with you about the pharmaceutical work. I had a conversation this morning with some of my collaborators, they're still at Brown. We discussed that this is completely lacking in understanding of epigenetic effects. There is very little data out there. It could be some of the most basic pharmaceuticals that people are taking during pregnancy that could actually be having huge effects and that no one is really looking into or thinking about. For example, there are a lot of people on antidepressants during pregnancy, or other medications for mental disorders. People still are take corticosteroids during pregnancy and often for good reasons. They themselves need it for their conditions. But I think we're not thinking about what that's doing to subsequent generations. I actually work with a pediatric HIV/AIDS cohort study, and epigenetic effects are something we're starting to look into now. I have a project with these cohorts thinking about epigenetic effects of the antiretroviral therapy. The pregnant women need these drugs. They're absolutely critical, and they're really lifesaving drugs that are preventing the transmission of HIV from mother to infant, but that infant is being exposed to this very potent drug. So it's not always clear what the effects of those might be in the long term. Mostly, they're safe, but we really don't know. This is an entirely new generation of children exposed to something new that are growing up now. Assisted reproductive technology is another great example of that. We're just starting to see what the possible effects could be because you now have the first in-vitro children getting to an age where they might be starting experiencing some of those effects and passing those effects on to the next generation. We're learning about that now. In the meantime, my feeling is if we're going to prenatally expose people to drugs, at least can we give them the opportunity upon reaching adulthood to know about it? Given that we're learning so much more about environmental epigenetics and long-term impacts, don't we at least want to give people the opportunity to have access to that medical information? I'm not saying you have to send them their files, but say, "Hey, you have some medical history. You may want to know about it." If you don't, that's your choice. And being able to at least provide some monitoring to these people. That's the nice thing with this HIV/AIDS cohort. At least they're following these children. There's an opportunity that if something does suddenly appear, actions can be taken. Otherwise, you just have people walking off with their life and maybe don't know what they've been exposed to and don't know that they should be concerned in any way. That's where I think it will be very important to make sure that we are at least providing that kind of monitoring to people that if we don't know what the effects are, let's at least keep track so that we could know. Our work in our children center here is looking at arsenic exposures because many people here use well water, and it can have very high arsenic levels. We're trying to get local pediatric practices to ask parents, "Have you had your well tested?" Getting them to think of that and adding that question to the list when they're talking to parents is a challenge as there is so much to think and talk about. I am interested in your work in behavioral outcomes. Would you mind talking about exposures that can have long-term behavioral outcomes? Sure. I think we know a lot about some toxic metals that can have this type of effect, like mercury and lead, and cadmium. What we're learning now and where our research interests are, is trying to understand what happens even at low level exposures. We know about some of these exposures at very high levels. You have these industrial accidents or extreme contamination of water then you see in children some behavioral effects, cognitive effects or changes in their quality of movement, things like that. We don't really understand what happens even at low levels that you might encounter here in the United States commonly or that we might have — for example, here in our well water. There may be elevated arsenic and what happens? Or even what might be considered safe levels. We don't really know what is safe and how low is safe. So I think that's an interesting area, just understanding even low level exposures. There's been great work in other children’s centers looking at pesticide exposures as well, as these chemicals have neurobehavioral effects. Coming to my last question -- dysregulation of imprinted genes may be causing these more pronounced abnormalities than you would have at, say, low level proximal fetal type of exposure. Absolutely. Yes, we actually are studying imprinting more on the somatic level. I think that mechanism is likely one of the most important because of the exquisite control that's needed of those genes. They have to be expressed at only a certain level, otherwise if they are too high or too low, you can have effects. So I absolutely think that that is a key mechanism. And because those are set in the germline, it's absolutely something that could be altered and with these types of exposures, could definitely be playing an important role. And those pass on. Those go from the germline and that could affect all the somatic cells of the body as they develop in subsequent generations. Even subtle effects can have a big impact. The focus of one of our projects now is looking at those subtle variations in imprinted expression, trying to link that to some behavioral outcomes. Oh, let’s keep in touch about that! I’m definitely excited about it. We think that because they are so exquisitely controlled, that any minor variation might be really important. It’s been wonderful talking with you, I was doing backflips reading about all your work because while many researchers are narrowly focused on one thing, you seem to have a bigger-picture view which I think is so important. Looking at these mechanisms and a wide range of outcomes is really important, and I think we can learn a lot more that way. Indeed. Thank you so much for your time today. Thank you very much. |