Overview of Human Germ Cell Developmental Stages and Critical Windows
By Meeri Kim, PhD
|
Humans have two primary types of cells. Germ cells are those involved in reproduction and include the well-known male and female gametes – egg and sperm, respectively – as well as their developmental precursors. By definition, somatic cells make up the remainder of an organism’s body such as skin, bones, organs, and blood.
Germ cells are essential to ensure the correct passage of DNA from one generation to the next. They represent the molecular phase of human development, with their own complex life cycle. It begins with the setting aside of primordial germ cells in the early embryo (specification) and ends with sperm and egg fusing together to begin a new life (fertilization). Throughout this life cycle, which generally lasts up to 40 years, there are several time-sensitive intervals during which exposures or events can disrupt development. These so-called critical windows leave the embryo vulnerable to adverse and irreversible epigenetic effects. What follows is a brief outline of the stages of germ cell development with critical windows highlighted. |
During the germline lifecycle there are several time-sensitive intervals during which exposures or events can disrupt development.
|
|
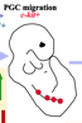
1. Specification
The germ cell lineage is one of the first to form in the human embryo. During early embryonic development, a small portion of cells are set aside through inductive signaling to later become germ cells. This process is termed specification, and at this early stage, they are called primordial germ cells (PGCs) and are located outside the embryo. In humans, PGCs are formed in the second week of development. In this period, they are thought to be epigenetically similar to the somatic cells around them. |
Human primordial germ cells are formed in the second week of embryonic development.
|
|
6. Germline through Childhood
After birth in the female, the germ cells are kept in a suspended state – meiotic arrest in prophase I – for many years, up to decades. They remain more or less unchanged from early embryogenesis until they are used for ovulation in the adult. During this period, any damage due to adverse environmental exposure could accumulate. Also, the total number of primary oocytes continues to decline during childhood until they number at about 400,000 right before puberty. Similarly, the male spermatogonia lie in wait until puberty and do not multiply. But the difference is that these cells have not lost their ability to multiply, and begin to do so again at puberty. |
During this period, any damage due to adverse environmental exposure could accumulate.
|
|
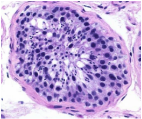
7. Germline in Puberty
In both sexes, mature germ cells are not fully formed until puberty. In females, through a process called folliculogenesis, the primary oocyte completes its first meiotic cycle and extrudes the first polar body at ovulation. In males, the spermatogonia activate and start to proliferate. Differentiation is yet another critical window, where DNA is re-packed and sensitive to environmental factors. Morphologically mature spermatozoa are produced by spermatogonia meiosis in the seminiferous tubules of the testes – a process that takes about 48 days. A healthy human male produces somewhere between 45 and 207 million spermatozoa per day that are then released into the central lumen of the seminiferous tubule. |
A healthy human male produces somewhere between 45 and 207 million spermatozoa per day that are then released into the central lumen of the seminiferous tubule.
|
|
9. Pre-Implantation
For the next few days, the zygote travels down the Fallopian tube and divides mitotically. First, it becomes a solid mass of cells called a morula. This stage is followed by the formation of a cavity within the ball. The blastocyst, as it is now known, contains an inner group of cells – to become the embryo – and an outer layer that will form protective membranes. Embryos during the morula/early blastocyst stage are considered to bear much lower levels of epigenetic marks than zygotes. The period of blastocyst formation includes extensive epigenetic reprogramming of the parental genomes, including genome-wide DNA demethylation. Once the blastocyst reaches the uterus, around week 4 of gestation, it implants itself tightly to the uterine wall and begins to receive nourishment from the mother’s blood. Around the time of implantation, the reprogramming process is largely complete. |
The period of blastocyst formation includes extensive epigenetic reprogramming of the parental genomes, including genome-wide DNA demethylation.
|