|
National Toxicology Program Office of Health Assessment and Translation (OHAT) Division of National Toxicology Program (NTP) National Institute of Environmental Health Sciences US Department of Health and Human Services Research Triangle Park, NC Submitted via NTP website December 11, 2018 Re: Nomination for scoping review of human and mammal evidence for adverse heritable impacts of direct germ cell exposure to drugs and chemicals Dear OHAT: In June of 2018 OHAT published a scoping review, “Human and animal evidence of potential transgenerational inheritance of health effects: An evidence map and state-of-the-science evaluation” (Walker et al., 2018). The immensely wide scope of the transgenerational report, the difficulty searching the literature, and the complexity of summarizing such a wide body of evidence—looking at different exposures, in different species, in different sexes, at different windows of developmental vulnerability, and with a plethora of different endpoints—surely made for a monumental undertaking. The undertaking seemed particularly difficult with regard to human exposures, considering that in most cohorts under study, insufficient time has passed to identify both F0 exposure and F3 outcomes. But more important, jumping to potential transgenerational effects without first ascertaining the state of the science on intergenerational inheritance induced by direct exogenous exposures to germ cells seemed hasty and premature—why concentrate on unexposed progeny while entirely skipping over the directly exposed (as a germ cell) offspring? This was a curious choice, especially in light of the fact that the American system of chemical and drug regulation currently ignores nearly all questions relating to direct germ cell impacts, creating a glaring need for scientific attention to this notably vulnerable window. This nomination asks OHAT to fill the void w ith a new scoping review devoted to questions of heritable (direct germ cell) effects of drugs and chemicals in human and mammal studies. Rationale for scoping review The second half of the 20th century witnessed a tsunami of novel chemical and pharmaceutical compounds, with countless newly devised substances entering the human body—and its germ cells—for the first time in the history of man. Beginning in the 1950s, scientists expressed alarm about the potential heritable force of some of these chemicals, leading to new approaches in toxicology as well as the founding of the Environmental Mutagenesis and Genomics Society (EMGS) in 1969 (Frickel, 2004; Wassom et al., 2010). Building on the original fears of the EMGS founders, the 21st century has seen a dramatic expansion of notions of inheritance to encompass molecular perturbation of germline by exogenous substances. While classic germ cell mutagenesis remains a stark concern in some cases (DeMarini, 2012), it is increasingly clear that exposures can induce adverse heritable effects quite apart from shifts in the DNA sequence—through the environmentally malleable layers of the epigenome of the germ cells (reviewed in Kexin et al. 2018; Skvortsova et al., 2018; Bonduriansky et al., 2018; Nilsson et al., 2018; Gapp et al., 2018; De Felici, et al., 2018; Marczylo et al., 2016). Indeed, a review of the scientific record reveals more than 100 studies in humans and mammals demonstrating adverse heritable effects of direct germ cell exposures (Escher, 2018b). None of this evidence of direct impact—which carries profound implications for understanding today’s public health challenges—was contemplated or included in the OHAT transgenerational scoping review of June of 2018. The American populace has been experiencing unprecedented rates of many serious pathologies, most of which cannot be explained by classic genetic or somatic environment paradigms. For example, the prevalence of autism, a serious lifelong neurodevelopmental disorder which entails vast societal cost, has surged to the point that 1 in 59 children are now identified as having autism spectrum disorders (Baio et al., 2018), with a prevalence observed to increase markedly with births in the 1980s (Nevison et al., 2018). Attention deficit hyperactivity disorder (ADHD) now affects 6.1 million children ages of 4 to 17, up from 4.4 million in 2003 (CDC, 2016). Obesity rates have surged, from a rare condition decades ago to 39.6% of adults in 2015-16 (Hales et al., 2018). Similarly, diabetes rates have surged dramatically (CDC, 2017). Sperm concentration and count have been dropping (Levine et al., 2017). The prevalence of polycystic ovary syndrome is as high as 15%–20% of women of childbearing age (Sirmans et al., 2014). The incidence of hypospadias has mysteriously doubled since the 1970s (Paulozzi et al., 1997). This is just a sample; many other conditions have been observed to be increasing in prevalence, with conventional “genetic” or “environment” approaches often unable to explain the numbers. Non-genetic inheritance caused by direct toxicant contamination of the germline may be a biological force driving up these mysteriously rising rates. If we apply contemporary understanding of germ cell toxicant vulnerabilities to pregnancy drug and chemical exposures of the postwar decades, one may reasonably ask whether today’s pathologies may be linked in part to those early, if forgotten, molecular interactions. For example, did maternal smoking, which reached its height in the 1960s, increase the risk for asthma, allergies, ADHD, and autism in grandchildren via exposure to the parents’ nascent germ cells? Several studies in mammals and humans suggest this may be true (Zhu et al., 2014; Rehan et al., 2013; Maritz et al., 2014, Golding et al., 2017; Accordini et al., 2018; Magnus et al., 2015; Lodge et al., 2018). Did synthetic steroid hormone drug exposure to fetal germ cells increase the risk for urogenital or neurodevelopmental abnormalities in the grandchild generation borne of the contaminated cells? Studies on diethylstilbestrol, for example, suggest this could be the case (Kioumourtzoglou et al., 2018; Tournaire et al., 2016). Does exposure to potent agents of general anesthesia damage primordial germ cells in a way that raises risks for learning and behavioral pathologies in the next generation offspring? Three published studies have demonstrated learning and behavioral impairment in the generation exposed as germ cells (Ju et al., 2018; Chalon et al., 1981; Tang et al., 1985). And countless other questions loom large. For example, did DDT and other pesticides quietly raise the risk for metabolic abnormalities in a generation borne of exposed germ cells? What about dioxin, chemotherapeutic agents, valproic acid, to name just a few? The evidence in the record thus far suggests these intergenerational questions are of tremendous importance to a full understanding of pathogenesis caused by exogenous toxicants. Moreover, the questions raised are not merely an exercise in retrospection. Every minute of every day, American children and adults ingest drugs and chemicals which may perturb the integrity of their germ cells, or, with respect to a pregnant woman, the integrity of her fetus’s germ cells as well. But risks to germ cells are routinely ignored in research, regulation, and practice. For example, if a man receives cortisone injections, there is no warning that the quality of his sperm may be temporarily affected. If a pregnant woman receives weekly injections of potent synthetic progestins, no one considers the potential impact on the fetal germline. If a woman is smoking tobacco, she is told of risks to the fetus, but not to her future grandchildren. If a couple visits a genetic counselor about their future offspring, the counselor will not even think to ask if either of them had been exposed to tobacco, synthetic steroids, or general anesthesia in utero. The United States is mired in archaic thinking about heritability, focusing solely on nucleotide sequence, while almost systematically ignoring germline vulnerabilities to toxicants. An OHAT scoping report on this topic could help change the national dialogue about pathogenesis of disease and disorder. It would examine the state of the evidence for intergenerational inheritance associated with direct germline exposure to chemicals and drugs in humans and mammals. By assessing the evidence for this form of non-genetic inheritance, it would help identify key areas of concern for public health and make recommendations for further research. Exposures to include in the scoping review This nomination seeks a review of research on heritable germline impact of exogenous chemicals and drugs. While other stressors such as diet, starvation, stress, and trauma could be considered as background material, this nomination is limited to exogenous chemicals and drugs (pharmaceutical and recreational, including tobacco and alcohol) only. The germline literature already includes research on the following:
For a list of human and mammal studies identified to date by the undersigned, please see germlineexposures.org (Escher, 2018b). Gametogenesis windows of vulnerability; mechanisms of interest It is clear that germline molecular impacts of toxicant exposure are highly dependent on the gametogenic window and sex of the germ cell. This introduces complexity to the present nomination, since analyses would need to be segregated by both timing of exposure and the sex of the exposed germ cell. Further complicating matters, germ cell exposures often yield sex-specific phenotypic outcomes in the progeny (see, for example, Ju et al., 2018; Krishnan et al., 2018; Golding et al., 2017). Background biological information is warranted. The epigenome refers to a heritable layer of biochemical information that includes DNA methylation, histone modifications, and non-coding RNAs. This information goes through exquisite reprogramming during the fetal germline phase. Because of epigenomic resetting, the fetal germ cells are at heightened sensitivity to perturbation by exogenous toxicants, which can act directly or indirectly to effectuate alterations in DNA methylation, histone modification and/or ncRNA expression (Gold et al., 2018; Marczylo et al., 2016). Abnormal hormonal signals, which may differ from endogenous signaling in terms of molecular structure, half life, and potency, can block, hyperactivate or otherwise disrupt normal hormone receptor function, ultimately influencing epigenomic regulation of gene expression (Marczylo et al., 2016). Because of these phenomena, “[e]pigenetic marks generated within germ cells as a result of environmental influences throughout life can also shape future generations long before conception occurs” (Bale, 2015). Below is an overview of commonly referenced critical windows, by sex. Note: the gestating woman is called “F0,” her exposed fetus is called “F1,” and the grandchildren borne of exposed fetal germ cells are called “F2.”  Exposures to female germ cells 1. Exposure of female F1 fetal germ cells via gestating F0. The male or female F1’s directly exposed embryonic/fetal germ cells later yield male or female grandchildren, or F2. Because embryonic/fetal germ cells are highly dynamic featuring multiple levels of reprogramming, as discussed above, this stage is considered an important critical window for exposure effects. 2. Exposure to a neonate female germ cells. Because the delicate process of genomic imprinting continues in the female ovary well after birth and through the first year, the infancy of the female may also present a critical window for exposures, though the molecular impacts are likely to be different than those seen in the primordial germ cell. 3. Exposure to germ cells of an adult pre-conception, non-gestating female. Some studies consider stressors to an adult female in the period well before or shortly before conception. The undersigned does not see much evidence for this window as a critical period of vulnerability for the egg epigenome, but is open to the possibility. That said, a pre-conception exposure of interest need not occur just before conception to be biologically relevant. A drug or chemical can be slow to metabolize, or may lodge in the fatty tissues even for decades (think of DDT or dioxin, for example). So long-gone exposures may still directly impact the contents of the egg that goes through the final stages of meiosis just prior to ovulation. Exposures to male germ cells 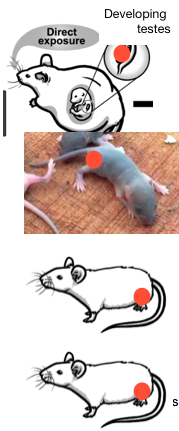 1. Exposure of male F1 fetal germ cells via gestating F0. This was discussed above. 2. Exposure to a neonate male germ cells. Similar to (2), above, except that the male germ cells have completed the imprinting process before birth. 3. Exposure to male germ cells during slow-growth period male. The period before puberty, when spermatogonia transition to spermatocytes. 4. Exposure to an adult pre-conception male germ cells. This is the final phase of spermatogenesis, the approximately 72 days in humans when primary spermatocyte matures into sperm. In sum, this nomination asks OHAT to undertake a scoping review of the state of the evidence for adverse heritable impacts of direct drug and chemical exposures to germ cells, in various stages of gametogenesis, but with a focus on the early germ cells which exhibit exceptional epigenomic vulnerability to toxicants. Again, an informal attempt at a first phase of this research, listing more than 100 human and mammal studies demonstrating direct germline effects, along with many reviews of the subject, has already been completed, with results published at germlineexposures.org (Escher, 2018b). As for endpoints, the literature suggests that neurodevelopment, neurobehavior, socio-sexual behaviors, and sexual development are often distorted as the result of germline exposure. Other endpoints of concern include asthma and allergy and metabolic dysfunction. Fifty years ago, leading scientists raised alarm bells about a possible ‘genetic emergency’ caused by the post-war influx of synthetic chemicals and drugs, concerned about subtle impairments in human germ cells that could affect the developmental integrity of future generations (Frickel, 2004; Escher, 2018a). Now that we understand germ cells not merely as an enclosure for a protein-coding template, but as complicated, highly dynamic biological entities that contain many layers of heritable information that can reshape gene expression and ultimately, brain development and behavior, it is time for taxpayer-funded research to embrace this biological reality, ascertain the risks, and take reasonable measures to safeguard the public. Thank you for your kind consideration of this nomination. Very truly yours, Jill Escher Cc: Vickie Walker, NIEHS Jack Bishop, NIEHS (ret) Jerry Heindel, NIEHS (ret) Linda Birnbaum, NIEHS Richard Woychik, NIEHS Francis Collins, NIH Diana Bianchi, NICHD Joshua Gordon, NIMH David DeMarini, EPA References Accordini S, Calciano L, Johannessen A, Portas L, Benediktsdóttir B, Bertelsen RJ, Bråbäck L, Carsin AE, Dharmage SC, Dratva J, et al. 2018. A three-generation study on the association of tobacco smoking with asthma. Int. J. Epidemiology;dyy031, https://doi.org/10.1093/ije/dyy031). Baio, J, Wiggins, L, Christensen, DL, Maenner, MJ, Daniels, J, Warren, Z, Kurzius-Spencer, M, Zahorodny, W, Rosenberg, CR, White, T, et al. 2018. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ 67:6;1–23. Bale, T. Epigenetic and transgenerational reprogramming of brain development. 2015. Nat Rev Neurosci 16;332–344. Bonduriansky R, Day T. 2018. Extended Heredity. Princeton, NJ: Princeton University Press. Chalon J, Tang CK, Ramanathan S, Eisner M, Katz R, Turndorf H. 1981. Exposure to halothane and enflurane affects learning function of murine progeny. Anesth Analg 60:794–7. DeMarini, DM. 2012. Declaring the Existence of Human Germ-CellMutagens. Environ Mol Mutagen 53:166-172. Escher J. 2018a. Bugs in the program: can pregnancy drugs and smoking disturb molecular reprogramming of the fetal germline, increasing heritable risk for autism and neurodevelopmental disorders? Environ Epigen 4:2;dvy001. Escher, J. Germline Exposures, Human and mammal studies finding heritable effects of exposures. 2018b. http://www.germlineexposures.org/studies-of-interest.html. Frickel S. 2004. Chemical Consequences: Environmental Mutagens, Scientist Activism, and the Rise of Genetic Toxicology. New Brunswick, NJ: Rutgers University Press p 87. Gapp K, Bohacek J. 2018. Epigenetic germline inheritance in mammals: looking to the past to understand the future. Genes Brain Beh 17:3,e12407. Gold HB, Jung YH, Corces VG. 2018. Not just heads and tails: the complexity of the sperm epigenome. J Biol Chem 293:36;13815-13820. Golding J, Ellis G, Gregory S, Birmingham K, Iles-Caven Y, Rai D, Pembrey M.l. 2017. Grand-maternal smoking in pregnancy and grandchild’s autistic traits and diagnosed autism. Sci Rep 7:46179. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007-2008 to 2015-2016. 2018 JAMA;319(16):1723-1725. Ju LS, Yang JJ, Morey TE, Gravenstein N, Seubert CN, Resnick JL, Zhang JQ, Martynyuk AE. 2018. Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats. Brit J Anesth 121:2;406-4168. Kexin Z, et al. Current Advance in Research of Gamete and Embryo-fetal Origins of Adult Diseases. Science China Life Sciences 2018 10.1007/s11427-018-9427-4. Kioumourtzoglou M, Coull BA, O’Reilly ÉJ, Ascherio A, Weisskopf MG. Association of Exposure to Diethylstilbestrol During Pregnancy With Multigenerational Neurodevelopmental Deficits. 2018 JAMA Pediatr 172:7;670-677. Krishnan K, Nitish Mittal N, Thompson LM, Rodriguez-Santiago M, Duvauchelle CL, Crews D, and Gore AC. Effects of the Endocrine-Disrupting Chemicals, Vinclozolin and Polychlorinated Biphenyls, on Physiological and Sociosexual Phenotypes in F2 Generation Sprague-Dawley Rats. 2018 Env. Health Perspect. https://doi.org/10.1289/EHP3550. Levine H, Jørgensen N, Anderson M-A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH. Temporal trends in sperm count: a systematic review and meta-regression analysis. 2017 Hum Reprod Update 23:6;646–659. Lodge CJ, Bråbäck L, Lowe AJ, Dharmage SC, Olsson D3, Forsberg B. 2018. Grandmaternal smoking increases asthma risk in grandchildren: a nationwide Swedish cohort. Clin. Exp. Allergy 48;167–74. Magnus MC, Håberg SE, Karlstad Ø, Nafstad P, London SJ, Nystad W. 2015. Grandmother’s smoking when pregnant with the mother and asthma in the grandchild: the Norwegian Mother and Child Cohort Study. Thorax 70:237-243. Marczylo EL et al, Environmentally induced epigenetic toxicity: potential public health concerns. Crit. Rev. Toxicol. 2016;46:8,676-700. Maritz GS, Mutemwa M. 2014. The effect of grand maternal nicotine exposure during gestation and lactation on lung integrity of the F2 generation. Pediatric Pulmonol 49:1;67-75. Nevison C, Blaxill M, Zaharodny W. 2018. California Autism Prevalence Trends from 1931 to 2014 and Comparison to National ASD Data from IDEA and ADDM. J Aut Dev Disord 48:12;4103–4117. Nilsson, EE, et al. Environmentally induced epigenetic transgenerational inheritance of disease. Environ Epigenetics. 2018;4(2). Paulozzi LJ, Erickson JD, Jackson RJ. 1997. Hypospadias trends in two US surveillance systems. Pediatrics 100:5;831-4. Rehan V, Liu J, Naeem E, Tian J, Sakurai R, Kwong K, Akbari O, Torday JS. 2012. Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med 10:129. Sirmans SM, Pate KA. 2014. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol 6:1–13. Skvortsova K, et al. Functions and mechanisms of epigenetic inheritance in animals. Nat Rev Molec Cell Biol 2018;19:774–790. Tang C-K, Chalon J, Markham JR, Ramanathan S.; Turndorf H. Exposure of Sires to Enflurane Affects Learning Function of Murine Progeny. Obstet Anesth Digest 1985;5:2,67. Tournaire M, Epelboin S, Devouche E, Viot G, Le Bidois J, Cabau A, Dunbavand A, Levadou A. 2016. Adverse health effects in children of women exposed in utero to diethylstilbestrol (DES). Therapie 71:4;395-404. U.S. Centers for Disease Control. 2016. Attention-Deficit / Hyperactivity Disorder (ADHD) https://www.cdc.gov/ncbddd/adhd/data.html (accessed November 9, 2018). U.S. Centers for Disease Control, Division of Diabetes Translation. 2017 Long-term Trends in Diabetes. https://www.cdc.gov/diabetes/statistics/slides/long_term_trends.pdf (accessed November 9, 2018). Walker VR, Boyles AL, Pelch KE, Holmgren, SD, Shapiro, AJ, Blystone, CR, Devito, MJ, Newbold RR, Blain R, Hartman P, et al. 2018. Human and animal evidence of potential transgenerational inheritance of health effects: An evidence map and state-of-the-science evaluation. Environ International 115:48-69. Wassom JS, Malling HV, Sankaranarayanan K, Lu P-Y. 2010. Reflections on the origins and evolution of genetic toxicology and the environmental mutagen society. 2010 Environ Mol Mutagen 51:8-9;746-760. Zhu J, Lee KP, Spencer TJ, Biederman J, Bhide PG. 2014. Transgenerational Transmission of Hyperactivity in a Mouse Model of ADHD. J Neurosci 34:8;2768-2773.
0 Comments
Your comment will be posted after it is approved.
Leave a Reply. |
AuthorJill Escher, Escher Fund for Autism, is a California-based science philanthropist and mother of two children with severe autism, focused on the question of how environmentally induced germline disruptions may be contributing to today's epidemics of neurodevelopmental impairment. You can read about her discovery of her intensive prenatal exposure to synthetic hormone drugs here. Jill is also president of Autism Society San Francisco Bay Area. Archives
July 2021
Categories |
- Home
-
Expert Q&A
- Eva Jablonka Q&A
- Amander Clark Q&A
- Mirella Meyer-Ficca Q&A
- Janine LaSalle Q&A
- Dana Dolinoy Q&A
- Ben Laufer Q&A
- Tracy Bale Q&A
- Susan Murphy Q&A
- Alycia Halladay Q&A
- Wendy Chung Q&A
- Pradeep Bhide Q&A
- Pat Hunt Q&A
- Yauk and Marchetti Q&A
- Emilie Rissman Q&A
- Carol Kwiatkowski Q&A
- Linda Birnbaum Q&A
- Virender Rehan Q&A
- Carlos Guerrero-Bosagna Q&A
- Randy Jirtle Q&A
- Jerry Heindel Q&A
- Cheryl Walker Q&A
- Eileen McLaughlin Q&A
- Carmen Marsit Q&A
- Marisa Bartolomei Q&A
- Christopher Gregg Q&A
- Andrea Baccarelli Q&A
- David Moore Q&A
- Patrick Allard Q&A
- Catherine Dulac Q&A
- Lucas Argueso Q&A
- Toshi Shioda Q&A
- Miklos Toth Q&A
- Piroska Szabo Q&A
- Reinisch Q&A
- Klebanoff Q&A
- Denis Noble Q&A
- Germline in the News
- Science
- Presentations
- About Us
- Blog
Proudly powered by Weebly

 RSS Feed
RSS Feed