Epigenetic Effects of Prenatal Alcohol Exposure
"I can say with high confidence that molecular aberrations are passed on via the epigenome across generations where they can produce significant differences in neurodevelopment."
 Laufer in the lab.
Laufer in the lab.
Interview with epigeneticist Benjamin Laufer, PhD candidate, Molecular Genetics Unit, Department of Biology, Western University, London, Canada. Laufer studies the long-term epigenetic effects of prenatal alcohol exposure.
Interviewed by Jill Escher, March 2014
What do you study in your lab and how did you get interested in epigenetics?
My lab studies how neurodevelopment responds to the environment at a molecular level. Specifically, we examine fetal alcohol exposure, as it directly targets the epigenome, so by disrupting these important events we can learn a lot about how the environment shapes our brains development. We’re also interested in this topic since it represents a significant public health concern because many women continue to drink at either binge or moderate levels during pregnancy in North America and Europe.
My interest in epigenetics really sparked when I was an undergraduate and first came across this video (Nova Science: Epigenetics Explains Why Twins Are Not Identical). Since then I haven’t been able to take my eyes off the advances the field is making and its implications. Essentially geneticists have learned that genetic determinism is indeed dead and that the environment has a much larger role in shaping how our genome is expressed and even inherited across generations. This has lead to what I view as a golden age and science is only now beginning to adjust and accommodate to these molecular marks.
This website concerns germline impacts of exposures but you study somatic impacts of fetal alcohol exposure. What is the difference between studying germ cell and somatic cell epigenetics?
Currently, most of our effort is focused on categorizing the somatic impacts on the developing brain. However, my background is in genetics and molecular inheritance is our true research interest. This is striking to me because our examination of the somatic effects has lead me to realize that Fetal Alcohol Spectrum Disorder (FASD) is not only an epigenetic disease, but also results in long-term and maintained changes that are heritable not only across cell divisions within an individual, but it can be inherited across generations through the germline.
Interviewed by Jill Escher, March 2014
What do you study in your lab and how did you get interested in epigenetics?
My lab studies how neurodevelopment responds to the environment at a molecular level. Specifically, we examine fetal alcohol exposure, as it directly targets the epigenome, so by disrupting these important events we can learn a lot about how the environment shapes our brains development. We’re also interested in this topic since it represents a significant public health concern because many women continue to drink at either binge or moderate levels during pregnancy in North America and Europe.
My interest in epigenetics really sparked when I was an undergraduate and first came across this video (Nova Science: Epigenetics Explains Why Twins Are Not Identical). Since then I haven’t been able to take my eyes off the advances the field is making and its implications. Essentially geneticists have learned that genetic determinism is indeed dead and that the environment has a much larger role in shaping how our genome is expressed and even inherited across generations. This has lead to what I view as a golden age and science is only now beginning to adjust and accommodate to these molecular marks.
This website concerns germline impacts of exposures but you study somatic impacts of fetal alcohol exposure. What is the difference between studying germ cell and somatic cell epigenetics?
Currently, most of our effort is focused on categorizing the somatic impacts on the developing brain. However, my background is in genetics and molecular inheritance is our true research interest. This is striking to me because our examination of the somatic effects has lead me to realize that Fetal Alcohol Spectrum Disorder (FASD) is not only an epigenetic disease, but also results in long-term and maintained changes that are heritable not only across cell divisions within an individual, but it can be inherited across generations through the germline.
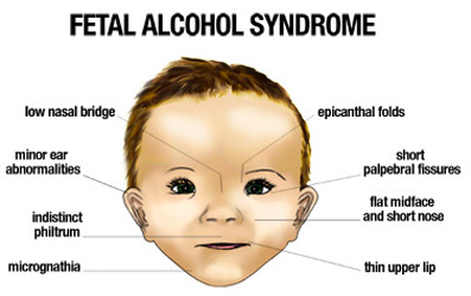 Laufer's work suggests FASD is a heritable, induced epigenetic disease.
Laufer's work suggests FASD is a heritable, induced epigenetic disease.
How do we know that neurodevelopment is shaped by epigenetic mechanisms?
This reminds me of my supervisor’s favorite analogy. The simplest explanation is a simple experiment. If we were to take just the genetic sequence of two parents and none of the epigenetic information (which is involved in gene regulation, cell-type differences, and 3D orientation of DNA) and place it in an egg, no life would form. This is because the real molecule of inheritance isn’t just the sequence of the DNA, which is a code of only 4 base pairs, but the chromatin molecule, which contains not only genetic sequence but an enormous code created by the epigenome that extends the code of life to a combination of hundreds of unique marks in millions of distinct places. Indeed, a good analogy for this is comparing the genome to computer hardware and the epigenome to computer software. Depending on what profile of marks you have on the DNA molecule, it can take on entirely different functions, like loading a different program onto a computer for different tasks.
Furthermore, even if we were to take a mass of cells with the proper initial epigenetic marks, a brain would not develop. This is because while ‘pure genetics’ determines some brain traits, it is not the only factor necessary for neurodevelopment; in fact, what’s most important and what truly shapes our brain is the interaction between our genetic content and the environment, which is carried out through the epigenome. This allows for a dynamic genome that can sense, respond, adapt to its surroundings, and incorporate that into its molecules of inheritance for future cells and generations.
What is epigenetics teaching us about risks of alcohol exposures that we didn't know from prior research on FASD?
Epigenetics has shown that the cause of fetal alcohol effects appears to originate at the molecular level by directly disrupting the epigenetic reprogramming that development depends on. The major implication for this is that because of the complexity of neurodevelopment, there are almost an infinite number of potential outcomes from fetal alcohol exposure, and there currently appears to be no defined lower threshold for these events.
You advocate a systems biology approach to understanding syndromes rather than a "reductionist" approach. The reductionist approach (eg, gene hunting) is the unfortunate status quo in autism research. What is your advice for the autism research world?
I think that all researchers of complex disease need to take a step back and look at the big picture. We are long past the era of simple single gene (Mendelian) diseases. Now we are dealing with complex multifactorial disease and related traits. They are caused by the interaction of large gene networks at both the genetic and epigenetic level. Essentially, every single case has the potential to be unique and is only grouped together as a single disease or disorder because of a similar outcome rather than a similar cause. Once we begin to understand the whole picture and all the variation occurring can we really focus in on a few candidates for clinical diagnosis and treatment.
What epigenetic mechanisms might be mediating the long-term effects of early ethanol exposures?
The current theory is that aside from the immediate toxicity caused by fetal alcohol exposure and its toxic acetaldehyde metabolite, there is also a more subtle and long-term effect. The major suspect is the one-carbon metabolism pathway, since that is where the methyl groups for DNA methylation and histone modification are donated. Alcohol exposure is known to interfere with this pathway and also obstruct the transfer of folate (a key vitamin in the pathway) across the placenta. So it appears that while methylation-related processes are the initial targets, because the epigenome is so interdependent on itself, this goes on to cause global changes.
Should the epigenetics paradigm change our thinking about the risks of gestational alcohol consumption?
Yes, I strongly think it should. While most women are aware of the effects of binge drinking during pregnancy, the public knowledge really falls behind when it comes to moderate exposures and the resulting umbrella term, fetal alcohol spectrum disorders (FASDs). There is compelling molecular evidence from our lab, and many others, that even moderate alcohol exposure during pregnancy produces significant negative outcomes in a child’s neurodevelopment and related function. In this case, there is no black and white, no defined minimal amount required for affect or safe time window in pregnancy. Potentially a single drink at the wrong moment could change a child’s life.
The real issue here is misinformation that is perpetuated by clinicians and the media, who are often not informed of the latest scientific findings and often misinterpret them due to the high level of specialization required. This is evidenced by all the sensationalized epidemiology studies observing no effect of moderate prenatal alcohol exposure – not because it’s not there, but because the methods aren’t sensitive enough to detect it – and then arriving at the improper conclusion that moderate fetal alcohol exposure is safe. Indeed, FASDs continues to be the leading cause of preventable mental retardation, affecting 2-5% of pregnancies, because a large amount of women are still under the antiquated impression that low-to-moderate alcohol exposure is safe for their unborn child.
This reminds me of my supervisor’s favorite analogy. The simplest explanation is a simple experiment. If we were to take just the genetic sequence of two parents and none of the epigenetic information (which is involved in gene regulation, cell-type differences, and 3D orientation of DNA) and place it in an egg, no life would form. This is because the real molecule of inheritance isn’t just the sequence of the DNA, which is a code of only 4 base pairs, but the chromatin molecule, which contains not only genetic sequence but an enormous code created by the epigenome that extends the code of life to a combination of hundreds of unique marks in millions of distinct places. Indeed, a good analogy for this is comparing the genome to computer hardware and the epigenome to computer software. Depending on what profile of marks you have on the DNA molecule, it can take on entirely different functions, like loading a different program onto a computer for different tasks.
Furthermore, even if we were to take a mass of cells with the proper initial epigenetic marks, a brain would not develop. This is because while ‘pure genetics’ determines some brain traits, it is not the only factor necessary for neurodevelopment; in fact, what’s most important and what truly shapes our brain is the interaction between our genetic content and the environment, which is carried out through the epigenome. This allows for a dynamic genome that can sense, respond, adapt to its surroundings, and incorporate that into its molecules of inheritance for future cells and generations.
What is epigenetics teaching us about risks of alcohol exposures that we didn't know from prior research on FASD?
Epigenetics has shown that the cause of fetal alcohol effects appears to originate at the molecular level by directly disrupting the epigenetic reprogramming that development depends on. The major implication for this is that because of the complexity of neurodevelopment, there are almost an infinite number of potential outcomes from fetal alcohol exposure, and there currently appears to be no defined lower threshold for these events.
You advocate a systems biology approach to understanding syndromes rather than a "reductionist" approach. The reductionist approach (eg, gene hunting) is the unfortunate status quo in autism research. What is your advice for the autism research world?
I think that all researchers of complex disease need to take a step back and look at the big picture. We are long past the era of simple single gene (Mendelian) diseases. Now we are dealing with complex multifactorial disease and related traits. They are caused by the interaction of large gene networks at both the genetic and epigenetic level. Essentially, every single case has the potential to be unique and is only grouped together as a single disease or disorder because of a similar outcome rather than a similar cause. Once we begin to understand the whole picture and all the variation occurring can we really focus in on a few candidates for clinical diagnosis and treatment.
What epigenetic mechanisms might be mediating the long-term effects of early ethanol exposures?
The current theory is that aside from the immediate toxicity caused by fetal alcohol exposure and its toxic acetaldehyde metabolite, there is also a more subtle and long-term effect. The major suspect is the one-carbon metabolism pathway, since that is where the methyl groups for DNA methylation and histone modification are donated. Alcohol exposure is known to interfere with this pathway and also obstruct the transfer of folate (a key vitamin in the pathway) across the placenta. So it appears that while methylation-related processes are the initial targets, because the epigenome is so interdependent on itself, this goes on to cause global changes.
Should the epigenetics paradigm change our thinking about the risks of gestational alcohol consumption?
Yes, I strongly think it should. While most women are aware of the effects of binge drinking during pregnancy, the public knowledge really falls behind when it comes to moderate exposures and the resulting umbrella term, fetal alcohol spectrum disorders (FASDs). There is compelling molecular evidence from our lab, and many others, that even moderate alcohol exposure during pregnancy produces significant negative outcomes in a child’s neurodevelopment and related function. In this case, there is no black and white, no defined minimal amount required for affect or safe time window in pregnancy. Potentially a single drink at the wrong moment could change a child’s life.
The real issue here is misinformation that is perpetuated by clinicians and the media, who are often not informed of the latest scientific findings and often misinterpret them due to the high level of specialization required. This is evidenced by all the sensationalized epidemiology studies observing no effect of moderate prenatal alcohol exposure – not because it’s not there, but because the methods aren’t sensitive enough to detect it – and then arriving at the improper conclusion that moderate fetal alcohol exposure is safe. Indeed, FASDs continues to be the leading cause of preventable mental retardation, affecting 2-5% of pregnancies, because a large amount of women are still under the antiquated impression that low-to-moderate alcohol exposure is safe for their unborn child.
 FASDs are the leading cause of preventable mental retardation.
FASDs are the leading cause of preventable mental retardation.
What should ordinary people know about subtle long-term effects of prenatal alcohol exposure?
They should know that Fetal Alcohol Syndrome (FAS) is just the tip of the iceberg and that any alcohol during pregnancy can produce FASDs. This outcome is not as noticeable as FAS, and potentially could affect a large amount of our population. It should also be known that while there is no cure, an early diagnosis is crucial. There are number of lifestyle changes that can be made to greatly improve a diagnosed child’s quality of life and help resume a more normal neurodevelopmental trajectory by providing an enriched environment for the epigenome to respond to while a child is still developing.
What are we learning about multigenerational impacts of fetal alcohol exposure?
They are there and it’s terrifying. I’ve seen multiple independent groups publish their molecular findings on it, and while I’m jealous they beat me to the punch, I can say with high confidence that molecular aberrations are passed on via the epigenome across generations where they can produce significant differences in neurodevelopment.
Interestingly, there is a distinction in the type of exposure that helps show the heritable nature of epigenetic marks. When a pregnant mother consumes alcohol, not only is she exposing her fetus directly to alcohol, but she is also exposing the precursor germline cells in her fetus that will go onto form the third generation. Because of this, there has been a strong interest in the field to distinguish between direct exposure (the first three generations) and indirect exposure (the fourth generation and after). It has been shown that these effects can be caused by indirect exposure and are thus truly inherited at the molecular level.
They should know that Fetal Alcohol Syndrome (FAS) is just the tip of the iceberg and that any alcohol during pregnancy can produce FASDs. This outcome is not as noticeable as FAS, and potentially could affect a large amount of our population. It should also be known that while there is no cure, an early diagnosis is crucial. There are number of lifestyle changes that can be made to greatly improve a diagnosed child’s quality of life and help resume a more normal neurodevelopmental trajectory by providing an enriched environment for the epigenome to respond to while a child is still developing.
What are we learning about multigenerational impacts of fetal alcohol exposure?
They are there and it’s terrifying. I’ve seen multiple independent groups publish their molecular findings on it, and while I’m jealous they beat me to the punch, I can say with high confidence that molecular aberrations are passed on via the epigenome across generations where they can produce significant differences in neurodevelopment.
Interestingly, there is a distinction in the type of exposure that helps show the heritable nature of epigenetic marks. When a pregnant mother consumes alcohol, not only is she exposing her fetus directly to alcohol, but she is also exposing the precursor germline cells in her fetus that will go onto form the third generation. Because of this, there has been a strong interest in the field to distinguish between direct exposure (the first three generations) and indirect exposure (the fourth generation and after). It has been shown that these effects can be caused by indirect exposure and are thus truly inherited at the molecular level.

