|
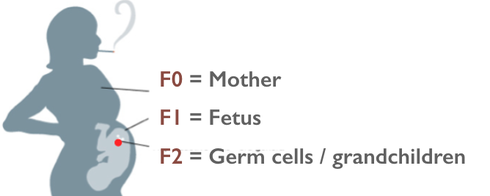
A few of the headlines after the study was published in Scientific Reports. While some headlines seemed a bit un-sober, it was gratifying to see the concept of fetal germ cell exposures hit the popular press. A pregnancy exposure affects two developing generations simultaneously: the fetus and the future grandchildren, via the exposed germ cells. A new UK study funded by the Escher Fund for Autism finds a grandmother's pregnancy smoking elevates risk of autism in grandchildren via the maternal line. By Jill Escher I'm excited to announce the results of a new study, "Grand-maternal smoking in pregnancy and grandchild’s autistic traits and diagnosed autism," published today in Scientific Reports. The study finds an increased risk of autism and autism-related traits in grandchildren, now about 26 years old, of women who smoked during pregnancy. The group studied is the ALSPAC Children of the 90s cohort, led by pioneering epidemiologists Jean Golding and Marcus Pembrey of the University of Bristol. Although the number of grandchildren with autism was small, around 170, the researchers found a notable increased risk of autism and autism-related traits when the grandmother had smoked cigarettes when pregnant with the mother (and, therefore, the mother's early eggs). See the University of Bristol's media release here for further details. Findings include that if a girl's maternal grandmother smoked during pregnancy, the girl is 67% more likely to display certain traits linked to autism, such as poor social communication skills and repetitive behaviors. Also, if the maternal grandmother smoked, this increased by 53% the risk of her grandchildren having a diagnosed autism spectrum disorder (ASD). The Escher Fund for Autism funded the study pursuant to its 2015 grant program, "20th Century Maternal Smoking: Induced Fetal Germline Perturbations in the Etiology of Autism and Neurodevelopmental Disorders.” A growing number of researchers are recognizing the importance of germline exposures, mutagenesis and epimutagenesis when assessing origins of neurodevelopmental pathology. "One of the important insights from this study is the implicit suggestion that modification of the genetic material of germ cells, in this case DNA of eggs, in grandmothers who smoked cigarettes during pregnancy can cause autistic phenotypes in the grandchildren," says Pradeep Bhide, PhD, Professor of Developmental Neuroscience at Florida State University. "Experimental animal models have suggested that epigenetic modification of germ cell DNA due to exposure to nicotine or other chemicals correlates with behavioral impairment in the descendants. Now this multi-generational human study underscores this point quite emphatically – in people." Personally, I see this study as something of a small ship that has struck a vast, unexplored continent. We are accustomed to thinking of autism risk in dualistic terms of genes or prenatal environment. In many cases, however, the answer may hinge instead on a third route: long-ago (and, alas, long forgotten) exposures disturbing elements within our germline genome or other germ cell components. Some of the many questions the study raises include: Do some genetic findings in autism have environmental roots? Perturbations to the germline may cause mutagenesis, epigenetic glitches or cytoplasmic error in the germline. Autism research tends to presume the great variety of genomic glitches seen in autism cases are "random," and that the heritability of autism somehow has natural roots, but has not yet investigated potential exogenous sources of those germline-borne errors. As Dr. Bhide says, "I hope that this new evidence will provide impetus to research on how exposure of the developing fetus to environmental influences, whether cigarettes, hormones or other chemical substances, that may have occurred in generations past can contribute to developmental disorders such as autism in the present generation, even in the absence of a genetic predisposition” Population and demographic effects? While enhanced risk may be relatively small on an individual level, the risk could be significant on a population level, given the large numbers of women who smoked during the latter half of the 20th century, particularly in western countries. and could help explain the timing of the autism increase, as well as the uneven socio-demographic patterns. Does pregnancy smoking also raise risk of autism in grandoffspring through the male line? This pilot study did not detect an association between grandmaternal tobacco use and autism risk through the fathers who had been exposed in utero but that may be due to the limited size of the study sample and not to any true lack of association. Many human and animal studies have demonstrated various deleterious impacts of tobacco on sperm, so this question remains worth pursuing in larger cohorts. A comprehensive literature review by Health Canada researchers is currently in press, examining the effects of smoking on sperm count and quality, chromosome and DNA damage, mutations, and potential impacts. According to the paper, the weight of evidence indicates that smoking impacts all of these parameters. They assessed the potential population level impacts of a modest increase in heritable mutations (25%). "Using 700 genes that are linked to intellectual disease, our model suggests that millions of individuals could be impacted by ID per generation globally as a result of Paternal Smoking," said Carole Yauk, PhD, Research Scientist in Genomics at Health Canada. This estimate is based on 2.4 million non-silent mutations in ID genes worldwide per generation: with 1.9 million being loss-of-function mutations. "This does not include the thousands of extra cases of aneuploid offspring that are likely because of paternal smoking, or deleterious mutations in genes associated with other disorders," she said. The researchers found that costs of this are on the order of $500 billion per generation (conservatively) for ID alone. (Aneuploidy refers to an abnormal number of chromosomes in a cell.) What are the relevant mechanisms of germline error and heritability? During fetal gametogenesis the molecular instruction book for the development of the following generation is in large part written. Research has shown early germline to be vulnerable to toxicants and environmental stressors during this period, affecting DNA, regulatory elements, epigenome, and cytoplasmic elements, including mitochondria. This study did not investigate mechanisms, but follow-up studies should address this question. Implications for regulatory review? Toxic, hormone-signal disrupting, and otherwise geno-affective pregnancy exposures increased dramatically in the post-war decades. These included the surge of maternal smoking and a great variety of synthetic pregnancy drugs. Every pregnancy exposure hits three generations at once: the mother (F0), the fetus (F1), and the future grandchildren (F2) via the exposed fetal germ cells. In spite of this plain biological reality, agencies such as the FDA have never assessed pregnancy drugs or tobacco for effects on the fetal germline or development of the F2 generation. (The Escher Fund for Autism has petitioned the FDA to change this policy of non-consideration of germline, for example here, but so far without effect.) Enduring effects on the subsequent generation? Animal models suggest multigenerational effects of gestational exposures to toxicants and endocrine disrupting chemicals. If tobacco smoke has destabilizing effects on human germline, do these pathological effects possibly endure for generations? This question could be of tremendous significance for autism families and others affected by the deleterious genomic effects of tobacco smoke. Can germline exposures like tobacco help explain the "broader autism phenotype"? Research shows that autism risk is elevated among siblings, but so is the "broader autism phenotype," which refers to related or subclinical traits associated with autism. The results of the ALSPAC study suggest tobacco toxicity could raise risk for both the pathology of autism and also the variety of traits associated with the disorder. What is the significance of doses, timing of smoking? Are only the grandoffspring of heavy (pack a day or more) smokers affected? According to some histories, "chain smoking" by women picked up only after World War II, owing the social factors, marketing and the costs of cigarettes. In addition, does timing of exposure matter? The early germ cell is molecularly vulnerable owing to the dynamic remodeling of the germline, including demethylation and the laying of imprints. The extent of genomic damage may hinge not only on dose but also timing of the exposure. What about other sources of fetal tobacco exposure? Might pre-pregnancy smoking also be significant for the fetus and germ cells, because some components of tobacco remain in maternal tissues long after the cessation of smoking? And what about second-hand smoke, which is also toxic? Further, of the 100s of toxic components of cigarette smoke, which one might be of most concern? For example, Benzo(a)pyrene? Nicotine? Why neurodevelopment? Of all possible phenotypic consequences of germline exposure, why does neurodevelopment seem to be particularly vulnerable? Research suggests a variety of possibilities: the hyper-mutability of long genes associated with neurodevelopment; impairment of genomic imprinting (lasting methylation of a subset of genes, many of which are associated with brain development and function); the role of ncRNAs; the sheer number of genes associated with brain function; and that genes that "escape" or resist epigenetic reprogramming relate to neurological function, among others. But this question was not addressed of course in this epidemiological study. Sex differences? Further examination is needed of differential sex effects on exposed F1 germ cell genome and epigenomic factors. Oogenesis and spermatogenesis feature many key differences in molecular and cellular processes and timing (girls are born with their eggs largely developed, boys are born with spermatogonial stem cells, which mature at puberty and regenerate sperm over many decades). Importantly, egg and sperm feature stark differences in cell physiology and components, which could influence effects of toxicant exposure. Furthermore, sex differences in F2 offspring autism rates and phenotypes warrants study. When it comes to heritable factors, egg and sperm are not equivalent. The egg contains vastly more material —and molecular information— carried into the next generation. Exposures during early oogenesis can affect that material. Sperm are also vulnerable through gametogenesis, but via somewhat different mechanisms.
Other pregnancy exposures? There are many additional fetal germline exposures of interest, including pregnancy drugs (for example, I, the mother of two children with severe idiopathic autism, was prenatally exposed to heavy and sustained combinations of synthetic steroid hormone drugs once popular in certain private clinics for ostensible prevention of miscarriage). While I have seed-funded some studies, larger wallets than mine are needed. Many thanks to the ALSPAC research team, especially Drs. Golding and Pembrey, for their assiduous and groundbreaking work, and undertaking this study to help to shed important new light on the forgotten histories that may be influencing autism risk through germ cells. This team has long been at the forefront of appreciating and demonstrating generational impacts. I also wish to thank all the wonderful Children of the 90s families — especially the autism families — for the immense gifts they continue to bestow to the rest of us. Related links: Germline Exposures (Escher Fund for Autism website) To Understand Autism, Talk to the Grandmothers (2017 commentary with background behind hypothesis) Autism's missing link? Study family history alongside genetics (2016 Autism Speaks blog) Out of the Past: Old Exposures, Heritable Effects, and Emerging Concepts for Autism Research (2016 presentation about the hypothesis) Germline Disruption Hypothesis of Autism (poster from 2016 research conference) Is grandmaternal smoking a force behind the autism surge? (2016 blog piece)
0 Comments
|
AuthorJill Escher, Escher Fund for Autism, is a California-based science philanthropist and mother of two children with severe autism, focused on the question of how environmentally induced germline disruptions may be contributing to today's epidemics of neurodevelopmental impairment. You can read about her discovery of her intensive prenatal exposure to synthetic hormone drugs here. Jill is also president of Autism Society San Francisco Bay Area. Archives
July 2021
Categories |
- Home
-
Expert Q&A
- Eva Jablonka Q&A
- Amander Clark Q&A
- Mirella Meyer-Ficca Q&A
- Janine LaSalle Q&A
- Dana Dolinoy Q&A
- Ben Laufer Q&A
- Tracy Bale Q&A
- Susan Murphy Q&A
- Alycia Halladay Q&A
- Wendy Chung Q&A
- Pradeep Bhide Q&A
- Pat Hunt Q&A
- Yauk and Marchetti Q&A
- Emilie Rissman Q&A
- Carol Kwiatkowski Q&A
- Linda Birnbaum Q&A
- Virender Rehan Q&A
- Carlos Guerrero-Bosagna Q&A
- Randy Jirtle Q&A
- Jerry Heindel Q&A
- Cheryl Walker Q&A
- Eileen McLaughlin Q&A
- Carmen Marsit Q&A
- Marisa Bartolomei Q&A
- Christopher Gregg Q&A
- Andrea Baccarelli Q&A
- David Moore Q&A
- Patrick Allard Q&A
- Catherine Dulac Q&A
- Lucas Argueso Q&A
- Toshi Shioda Q&A
- Miklos Toth Q&A
- Piroska Szabo Q&A
- Reinisch Q&A
- Klebanoff Q&A
- Denis Noble Q&A
- Germline in the News
- Science
- Presentations
- About Us
- Blog
Proudly powered by Weebly


 RSS Feed
RSS Feed