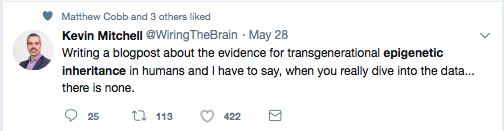
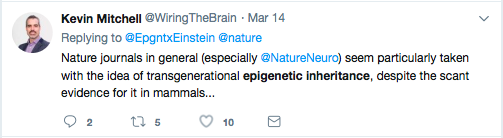
No Convincing Evidence? A Response to Kevin Mitchell’s Reckless Attack on Epigenetic Inheritance7/18/2018 By Jill Escher With no small amount of alarm I recently read Kevin Mitchell’s dismissal of the idea that environmentally informed nongenetic inheritance plays a role in the etiology of human traits and pathologies. I’m referring to his May 29, 2018 Wiring the Brain blogpost, “Grandma’s trauma – a critical appraisal of the evidence for transgenerational epigenetic inheritance in humans.” Now, I hardly mind critical appraisals of evidence in epigenetics or anything else, but the resounding sweep of his conclusions is so overstated and divorced from reality I felt I could not let it stand without comment. Mitchell, Associate Professor in Genetics and Neuroscience at Trinity College, Dublin, contends “there is no convincing evidence showing transgenerational epigenetic inheritance in humans.” His implication is clear: only “sociological reasons,” and not sound science, lie behind the continuing scientific and popular interest in this “sexy story,” which based on his listing of alleged sociological reasons seems to involve nothing more than an echo chamber of lazy researchers unnecessarily diverting attention from that King of Inheritance, determinism via the DNA sequence. There is nothing remotely subtle about Mitchell’s contempt for the science of epigenetic inheritance in mammals and humans. He states that the “idea that a person’s experiences can somehow mark their genomes in ways that are passed on to their children and grandchildren” then influencing “gene expression and affect[ing] the behaviour and physiology of people who inherit them” is “based on the flimsiest of evidence from a very small number of studies with very small sample sizes and serious methodological flaws.” Most alarmingly (at least to me), he scoffs at any implication of “epigenetics as a crucial new mechanism in medicine and public health – both a cause of disease and a potential therapeutic target.” While I won’t quibble with the “therapeutic target” part, the contention that epigenetic inheritance is unimportant for public health… well, as a prenatally-exposed-to-crud person myself, one who loudly champions the need for more research in this field (see “Bugs in the Program” in the journal Environmental Epigenetics), that hit me right in the gut. So I urge readers to reconsider his broadside. After all, scientific ideas have consequences. And since Mitchell’s post got a lot of TwitterLove, not to mention a stamp of approval from Jerry Coyne, I fear Mitchell’s words could, however subtly, dampen interest in an area I think deserves a rave-party level of enthusiasm. Much is at stake in this debate, including our hive-mind willingness to explore the heritable risks of pregnancy drugs, chemicals, smoking and trauma. "Much is at stake here, including our hive-mind willingness to explore the heritable risks of pregnancy drugs, chemicals, smoking and trauma." We pause for a moment of linguistic clarity There are many definitions floating around for “transgenerational,” “epigenetic” and related terms and phrases, and I hardly blame Mitchell for lexical mushiness seen in his and many other writings (including my own). But when he derides “transgenerational epigenetic inheritance,” I have difficulty figuring out what he actually means, from a biological point of view. “Transgenerational” usually refers to inherited traits without any direct exposure to the soma or germline. In contrast, “intergenerational” phenomena usually refer to heritable results of a direct exposure to the germline. Mitchell looks at studies investigating three cohorts: offspring and grand offspring of men and women living in Överkalix, Sweden (variable food supply); offspring of women gestating during the Dutch Famine (suffering often severe malnutrition); and grandoffspring of pregnant women in São Gonçalo, Brazil (exposed to violence). For the most part the phenomena at issue in these studies are not “transgenerational,” but rather “intergenerational.” So I’ll use that term. [Because these processes are clearly extremely sex- and developmental window- specific, a certain degree of biological precision is warranted. Appendix 1 provides a starter-kit outline for those who want to learn more.] Lamenting epigenetics hype in the popular media is cute, but irrelevant As an aside, and this hardly seems worth addressing, but Mitchell invokes media hype around epigenetics to heighten a dramatic contrast to supposedly “flimsy” findings in research. Sure, media hype happens. But his argument is like blaming researchers for the National Enquirer (or equivalent tabloid in Dublin) saying “Scientists Find Alien Civilization” when in fact they only reported locating an unusual array of ancient ruins on a remote island. Whining about media coverage is a straw man. It’s irrelevant to question of whether the scientific literature reveals evidence for epigenetic inheritance, or not. A brief peek into the biology of intergenerational inheritance As I mentioned above, intergenerational inheritance involves a direct stressor to the developing germline. How might that work? To offer an example of just one molecular mechanism, in the early germ cell, exogenous steroid or steroid-like molecules can bind to receptors (or exogenous substances can impede normal binding of endogenous steroids), which can translocate to the nucleus, and act directly on the genome as transcription factors. This may affect chromatin and/or how the DNA gets re-methylated in the germ cells, and after fertilization may cause changes in transcription in adult tissues, via either up- or down-regulation. This is not magical thinking or hocus pocus —as Mitchell seems to perceive it—but boring reproductive molecular biology. From a broader perspective, the development and reprogramming of early germ cells is overwhelmingly complex but appears to involve very precisely choreographed, and heavily evolutionarily conserved, activation and deactivation of many layers of molecular mechanisms, many of which are informed by hormonal or toxicological actions. Interference with this delicate process can persist in the germ cells and reveal themselves as traits or pathologies in the offspring. While Mitchell did not respond to my query to him about vulnerabilities of early germ cells, I imagine he might raise the point that any molecular mischief inflicted on a primordial germ cell is biologically moot due to post-conception epigenetic reprogramming in the form or demethylation and remethylation of the germline. Okay, but as I understand it some regions escape this process, including retrotransposons and some genes associated with metabolic and neurological disorders, and further, other mechanisms could remain in play, for example ncRNAs and histone marks. Long story short, the biology is pretty clear that early exposures can have persistent consequences through the germline. Mitchell cherry picks human studies Concerned about his cherry picking of human studies, I asked Mitchell via Twitter why he ignored human study evidence that diethylstilbestrol given in pregnancy (F0) caused intergenerational (F2) effects. He did not deny this evidence but seemed to backpedal, saying “I was focusing here on the literature claiming effects of social experiences from grandparents to grandchildren.” Putting aside his characterization of starvation as a “social experience,” it’s pretty clear that his blog did not make that distinction—to the contrary, it fist-pounded, “So, there you have it. In my opinion, there is no convincing evidence showing transgenerational epigenetic inheritance in humans.” Nor did he amend or clarify the blog after I brought this issue to his attention. So, no, I don’t believe that is what he meant.[fn] I, perhaps more than anyone, would like to see more studies of environmentally induced inheritance in humans. Why is there not more, though? First, multigenerational human epidemiological cohorts are hard to come by—three generations for intergenerational inquiries are rare, and four generations for transgenerational studies nonexistent. And second, human molecular studies are pretty much impossible due to ethical considerations and lack of access to the tissues of interest. As Mitchell must be aware, we can’t expose pregnant women to toxicants or starvation or whatnot and then extract their fetuses and their germ cells, or carve into their unconsenting babies’ or grandbabies’ brains or body parts. So obviously we must rely on animal models to enrich our understanding of mechanisms. But back to epidemiology, while I can understand some of Mitchell’s concerns about Överkalix, Dutch Famine, and São Gonçalo conclusions—sample size, study design, multiple testing corrections and “noise” in the data are valid points—there are no perfect human retrospective studies. Even with their imperfections, these carefully considered analyses offered early hints about germline-mediated generational effects of developmental-stage stresses, and have an important place in the history of epidemiology. No one ever said they should stand in for all of human epidemiology in this realm of inheritance. But now that it’s 2018, let’s please acknowledge other human studies that have found intergenerational effects of exposures, including the following. Kioumourtzoglou M, et al. Association of Exposure to Diethylstilbestrol During Pregnancy With Multigenerational Neurodevelopmental Deficits. JAMA Pediatr published online May 2018. This study of 47,450 women in the Nurses’ Health Study II found significantly elevated odds for ADHD in the grandchildren of women who took the toxic synthetic hormone drug DES (diethylstilbestrol) during pregnancy. [Yes, some studies I list in this response are new and I know Mitchell is not clairvoyant. Nevertheless they help paint the larger picture.] • Also see JAMA Peds commentary: Nigg, J Toward an Emerging Paradigm for Understanding Attention Deficit Hyperactivity Disorder and Other Neurodevelopmental, Mental, and Behavioral Disorders: Environmental Risks and Epigenetic Associations, JAMA Peds, published online May 2018. (“If disorders like ADHD are epigenetic conditions [that is, dependent on or heavily modulated by discoverable epigenetic changes that are traceable to preventable environmental exposures], it would have powerful implications for where national research dollars should focus to find ways to reduce the incidence of ADHD and other mental disorders.”) Golding J, et al. Grand-maternal smoking in pregnancy and grandchild’s autistic traits and diagnosed autism. Sci Rep 2017;7:46179. This study of the ALSPAC cohort found significantly higher risk of autism and autism-related traits in the grandchildren of women who smoked cigarettes during pregnancy, through the exposed females. Tournaire M, et al. Birth defects in children of men exposed in utero to diethylstilbestrol (DES), Therapie, March 3, 2018. The study suggests an increased incidence of two male genital tract defects in sons of men prenatally exposed to DES. This intergenerational effect had already been observed in animals and in the offspring of women prenatally exposed to DES. Kalfa N, et al. Prevalence of hypospadias in grandsons of women exposed to diethylstilbestrol during pregnancy: a multigenerational national cohort study. Fertil Steril 2011;95(8):2574-2577. This nationwide cohort study in collaboration with a French association of DES-exposed women studied 529 families and showed that a significant proportion of boys born to DES daughters exhibited hypospadias. Brouwers MM, et al. Hypospadias: a transgenerational effect of diethylstilbestrol? Hum Reprod 2006;21(3):666-669. An increased risk of hypospadias was observed when mothers were exposed to DES in utero. However, the excess risk appears to be of much smaller magnitude than in the 2002 study (below). Klip H, et al. Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet 2002;359(9312):1102-1107. Found an increased risk of hypospadias in the sons of women exposed to DES in utero, though the absolute risk was small. Titus-Ernstoff L, et al. Menstrual and reproductive characteristics of women whose mothers were exposed in utero to diethylstilbestrol (DES). Int J Epidemiol 2006;35 (4):862-868. Found menstrual irregularity and possible infertility in granddaughters of women who took DES in pregnancy. Titus-Ernstoff L, et al. Birth defects in the sons and daughters of women who were exposed in utero to diethylstilbestrol (DES). Int J Androl 2010;33:377–84. Data suggest a possible association between the mother’s prenatal DES exposure and birth defects in their offspring, particularly in daughters. We cannot, however, rule‐out the possible influence of reporting bias. In particular, the exposed daughters’ elevated risk of cardiac defects may be as a result of the underreporting of these conditions by unexposed mothers. Titus-Ernstoff L, et al. Offspring of women exposed in utero to diethylstilbestrol (DES): a preliminary report of benign and malignant pathology in the third generation. Epidemiology 2008;19:251–7. Based on a small number the risk of ovarian cancer was higher in daughters of women prenatally exposed to DES. Shnorhavorian, M, et al. Differential DNA methylation regions in adult human sperm following adolescent chemotherapy: potential for epigenetic inheritance, PloS One 2017;12(2)journal.pone.0170085. Adolescent chemotherapy exposure promoted epigenetic alterations that persisted approximately ten years after exposure. A signature of statistically significant DMRs was identified in the exposed males, found in CpG desert regions of primarily 1 kilobase size. This study did not investigate phenotypic outcomes in the next generation, but the topic, and suggestion of possible impairment of offspring, is of such tremendous social importance I felt the need to include it here. And here’s an interesting new one, though mechanisms of transgenerational effect are unknown: Patel B, et al. Transgenerational effects of chemotherapy: Both male and female children born to women exposed to chemotherapy have fewer children. Cancer Epidemiology October 2018;56:1-5. The sons and daughters (F1 generation) of chemotherapy-exposed women have fewer (74-77% fewer) live births when compared to both matched, unexposed general population and cousin controls. Of course we would benefit from more evidence of generational effects of exposures in humans. But to say “there is none,” zippo, nada evidence of intergenerational epigenetic inheritance (again, what he really meant by transgenerational), well, that’s just nuts, a reckless, unfair characterization of the science. But worse is his assertion that further research in the area is a waste of time—propped up only by sociological pressures. To the contrary, these studies are delectable hors d’oeuvres to what should be a meatier main course. Now is the time to intensify our inquiries: what are the heritable germ cell effects of pregnancy and neonatal general anesthesia? Of tobacco (aside from the study I cited)? Synthetic steroids aside from DES? Of psychoactive drugs? What about opioids or cannabis? Of heavy doses of industrial chemicals or pesticides? The list of ridiculously important human exposures we should be studying could fill this entire blog. It fairly terrifies me that an influential academic seems to consider these lines of inquiry unimportant. Mitchell ignores abundant evidence of epigenetic inheritance in mammals Okay, this part really got me. Mitchell admits there is very good evidence for epigenetic inheritance in nematodes and plants, and “in specific circumstances involving transposable elements in mice,” linking to a single 2006 study that examined mechanisms in a genetically induced model. Okay, but what about all those other mammal studies in the literature? He contends they “suffer from all the same methodological problems as these human studies, as I have previously discussed here and here" (linking to blog posts that hardly discuss mammal studies). No disrespect, but c’mon, Mitchell’s treatment of this subject is sloppy to the point of awful. Please permit me to share more than 30 of the studies supporting the existence of intergenerational epigenetic, or at least nongenetic (for those observing phenotypes but not mechanisms), inheritance in mammals. Mitchell has long been vocal with his antagonism toward the concept that exposures can have generational consequences through the germline epigenome. Agents of general anesthesia and analgesics Ju LS, et al. Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats, Brit J Anesth 2018. In a rat model, neonatal exposure to the widely used general anesthetic agent sevoflurane can affect the brains and behavior of the next generation of males through epigenetic modification of Kcc2 expression, while F1 females are at diminished risk. • Also See BJA editorial: Vutskits L, et al. A poisoned chalice: the heritage of parental anaesthesia exposure, Brit. J Anesth, 2018. (“Hence, we are faced with a real possibility that general anaesthetics are not innocuous agents that ‘only put children to sleep’ but rather formidable modulators of chromatin remodeling and function…. The current study extends previous reports of sex differences by showing that anaesthetic exposure itself can alter expression of chloride channels in certain brain regions and that this effect is heritable from exposed male parents to unexposed offspring.”) Chalon J, et al. Exposure to halothane and enflurane affects learning function of murine progeny. Anesth Analg 1981;60:794–7. In a mouse model, learning retardation was seen in offspring of murine parents exposed to GA in utero—in other words, mental impairment in the grandpups of the exposed gestating dams. Tang C-K, et al. Exposure of sires to enflurane affects learning function of murine progeny. Obstet Anesth Dig 1985;5:2, 67. In a mouse model, the general anesthetic agent enflurane administered to male mice was found to adversely affected learning function of their offspring. Rossitto, M, et al, Intergenerational effects on mouse sperm quality after in utero exposure to acetaminophen and ibuprofen. FASEB J. 2018. In a mouse model, demonstrates that pregnancy exposure to these analgesics during the critical period of sex determination affects the germ-line development and leads to adverse reproductive effects in the grandpups. Nicotine and tobacco Zhu J, et al. Transgenerational transmission of hyperactivity in a mouse model of ADHD. J Neurosci 2014;34:2768–73. In a mouse model, grandpups of gestating dams exposed to nicotine exhibited behaviors comparable to ADHD. Rehan VK, et al. Perinatal nicotine-induced transgenerational asthma. Am J Physiol Lung Cell Mol Physiol 2013;305:L501–7. In a rat model, grandpups of gestating dams exposed to tobacco smoke exhibited higher risk for asthma traits. Meier MJ, et al. In utero exposure to benzo[a]pyrene increases mutation burden in the soma and sperm of adult mice. Environ Health Perspect 2017;125:82–8. In a mouse model, higher mutation rates were found in offspring sperm when the pregnant dam was exposed to the tobacco component BaP. Okay slightly off topic, but let’s not forget the importance of de novo mutagenesis in germ cells, which can be precipitated by exposure to mutagenic substances such as BaP. Now back to our program. Synthetic steroids Moisiadis VG, et al. Prenatal Glucocorticoid Exposure Modifies Endocrine Function and Behaviour for 3 Generations Following Maternal and Paternal Transmission. Sci Rep 2017;7:11814. In a guinea pig model, gestational treatment with synthetic glucocorticoids (betamethasone) at a clinically relevant dose resulted in various generational pathology including altered cortisol response to stress, altered expression of genes in the prefrontal cortex and hypothalamic paraventricular nucleus. Transgenerational alterations of programming was seen through F3. Transmission was sex- and generation-dependent, occurring through both parental lines. Iqbal M, et al. Transgenerational effects of prenatal synthetic glucocorticoids on hypothalamic-pituitary-adrenal function. Endocrinology 2012;153, 3295–3307. In a guinea pig model, gestational treatment with synthetic glucocorticoids (betamethasone) modified HPA function and behavior in the F2. Long NM et al. Multigenerational effects of fetal dexamethasone exposure on the hypothalamic-pituitary-adrenal axis of first- and second-generation female offspring. Am J Obstet Gynecol 2013; 208, 217.e1–217.e8. In a sheep model, the synthetic glucocorticoid dexamethasone administed in the clinical range to gestating ewes have multigenerational effects on HPA activity. Drake AJ, et al. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol 2005;288, R34–R38. In a sheep model, pregnant ewes were exposed to the synthetic glucocorticoid dexamethasone, a variety of pathologies (reduced birth weight, glucose intolerance, and elevated hepatic PEPCK activity) were seen in male grandoffspring. Drake AJ, et al. Multigenerational programming in the glucocorticoid programmed rat is associated with generation-specific and parent of origin effects. Epigenetics 2011;6:1334–43 In a rat model, prenatal glucocorticoid overexposure caused effects on fetal and placental weight in both the F1 and F2 offspring, with marked parent-of-origin effects in F2. Vaughan OR, et al. (Dexamethasone treatment of pregnant F0 mice leads to parent of origin-specific changes in placental function of the F2 generation. Reprod Fertil Dev 2015;27(4):704-11. In a mouse model, effects of F0 gestating dam dexamethasone exposure are transmitted intergenerationally to the F2 placenta via the maternal, but not paternal, line. de Assis S, et al. High-fat or ethinyl-oestradiol intake during pregnancy increases mammary cancer risk in several generations of offspring. Nat Commun 2012;3:1053. In a rat model, fetal exposure to diets high in fat or a large amount of estrogen heightened the risk of breast cancer for three generations of female offspring. Epigenetic changes in the mammary glands of three generations of the rats who had been exposed to increased estrogen were observed. Horan TS, et al. Germline and reproductive tract effects intensify in male mice with successive generations of estrogenic exposure. PLOS Genetics 2017;1006885. In a mouse model, multiple generations of exposure not only exacerbate germ cell exposure effects, but also increase the incidence and severity of reproductive tract abnormalities. Environmental endocrine disruptors/pesticides/plasticizers Crews D, et al. Epigenetic transgenerational inheritance of altered stress responses. Proc Natl Acad Sci USA 2012;109:9143–8. In a rat model, a single exposure to vinclozolin altered the physiology, behavior, metabolic activity, and transcriptome in discrete brain nuclei in descendant males, causing them to respond differently to chronic restraint stress. Wolstenholme JT, et al. Gestational exposure to bisphenol A produces transgenerational changes in behaviors and gene expression. Endocrinology 2012;153:3828–38. In a mouse model, gestational exposure to BPA produces multigenerational alterations in genes and behavior. Drobná Z, et al. Transgenerational effects of bisphenol A on gene expression and DNA methylation of imprinted genes in brain. Endocrinology 2018;159:1132–144. In a mouse model, gestational exposure to BPA produces multigenerational alterations in brain tissues. BPA-caused changes at two imprinted genes in the brain were observed. Gely-Pernot, A, et al. Gestational exposure to chlordecone promotes transgenerational changes in the murine reproductive system of males Sci Rep 2018;8:10274. In a mouse model, pregnant females were exposed to chlordecone, an organochlorine insecticide. Subsequent generations suffered reduction in spermatogonia, meiotic defects in spermatocytes and decrease in spermatozoa number. Changes in the expression of genes associated with chromosome segregation, cell division and DNA repair were observed. Altered epigenetic marks were conserved between F1 and F3 generations. Skinner MK, et al. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One 2008;3:e3745. In a rat model, gestating females were exposed to the endocrine disrupting fungide vinclozolin during fetal gonadal sex determination. Alterations to epigenetic reprogramming of the male germ-line and offspring brain transcriptome (sex-specific) were observed, Several brain signaling pathways were influenced including those involved in axon guidance and long-term potentiation. Iqbal K, et al. Deleterious effects of endocrine disruptors are corrected in the mammalian germline by epigenome reprogramming. Genome Biol 2015;16:59. In a mouse model, gestating mice were treated with endocrine-disrupting chemicals vinclozolin, bisphenol A, or di-(2-ethylhexyl)phthalate, resulting in changes in transcription and methylation in the F1 germline. Though intergenerational changes were observed, transgenerational (no direct exposure) were not. Manikkam M, et al. Dioxin (TCDD) Induces Epigenetic Transgenerational Inheritance of Adult Onset Disease and Sperm Epimutations. PLoS One 2012:e46249. In a rat model, gestating females were exposed to dioxin, increasing the incidences of multiple diseases in subsequent generations, including kidney disease in males, pubertal abnormalities in females, ovarian primordial follicle loss and polycystic ovary disease. Analysis of the F3 sperm epigenome identified 50 differentially DNA methylated regions in gene promoters. Valproic acid Choi CS, et al. The transgenerational inheritance of autism-like phenotypes in mice exposed to valproic acid during pregnancy. Sci Rep 2016; 6:36250. In a mouse model, valproic acid (an anti-convulsant drug) induced epigenetic inheritance of autism-like neurobehavioural phenotype in mice through the paternal germline in first and second generation. Cipriani C, et al. High expression of Endogenous Retroviruses from intrauterine life to adulthood in two mouse models of Autism Spectrum Disorders. Sci Rep 2018 8(1):629 In a mouse model, findings of a transgenerational effect of prenatal valproic acid exposure. In the second and third generation, more marked transcriptional effects were seen in offspring from females, compared to paternal lineages. McBirney M, et al. Atrazine induced epigenetic transgenerational inheritance of disease, lean phenotype and sperm epimutation pathology biomarkers. Plos ONE 2017;12(9): e0184306. https://doi.org/10.1371/journal.pone.0184306 In a rat model, gestating females were exposed to atrazine. The F2 generation (grand-offspring) was found to have increased frequency of testis disease and mammary tumors in males and females, early onset puberty in males, and decreased body weight in females compared to controls. Chemotherapeutic agents Glen, CD, et al. Exposure to anticancer drugs can result in transgenerational genomic instability in mice. Proc Natl Acad Sci 2012;109(8):2984–2988. In a mouse model, paternal exposure to a commonly used chemotherapeutic agents resulted in increased mutation rates and transgenerational instability. (De novo mutagenesis, not epigenetic per se, was investigated.) Chan, D et al. Epigenetic alterations in sperm DNA associated with testicular cancer treatment. Toxicol Sci 2012;125(2):532–543. In a rat model, treatment with chemotherapeutic agent resulted in DNA methylation alterations in sperm. This study did not investigate resulting phenotype in offspring borne of the epigenetically altered sperm. Diet Dunn, GA et al. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology 2011;152, 2228–2236. In a mouse model, a high-fat diet in gestation resulted in larger F3 female offspring. A potential dynamic pattern of paternally expressed genes from the paternal lineage was seen at an imprinted locus, thereby providing sex specificity to both the transmission and inheritance of traits related to disease predisposition. Odor fear conditioning Dias, BG et al. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci 2014;17, 89–96. In a mouse model, pre-conception adult mice were subjected to odor fear conditioning, with behavioral, neuroanatomical, and epigenomic consequences in unexposed pups and grandpups. Immune activation Weber-Stadlbauer, U. et al. Transgenerational transmission and modification of pathological traits induced by prenatal immune activation. Mol Psychiatry 2017; 22:102-112. In a mouse model, prenatal immune activation by the viral mimetic poly(I:C) resulted in alterations in brain and behavioral functions in multiple generations. Reduced sociability and increased cued fear expression in first- and second-generation offspring were observed, in addition to other sex-specific effects. Genome-wide transcriptional changes were also seen. Chronic stress/traumatic experience Rodgers, AB, et al. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 2013;33:9003–9012. In a mouse model, males were exposed to six weeks of chronic stress before breeding. Offspring displayed significantly reduced HPA stress axis responsivity. Changes in transcription were seen in the brain, suggestive of epigenetic reprogramming and consistent with altered offspring stress responsivity, including increased expression of glucocorticoid-responsive genes in the PVN. In examining potential epigenetic mechanisms of germ cell transmission, robust changes in sperm microRNA were found. Rodgers, AB, et al. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci USA 2015;112,13699–13704. In a mouse model, after males were exposed to chronic stress, sperm miRNAs were found postfertilization to alter offspring stress responsivity. Also zygote microinjection of the miRs, demonstrated a recapitulation of the offspring stress dysregulation phenotype. Gapp, K. et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci 2014;17, 667–669. In a mouse model, traumatic stress in early life altered mouse microRNA expression, and behavioral and metabolic responses in the progeny. Injection of sperm RNAs from traumatized males into fertilized wild-type oocytes reproduced the behavioral and metabolic alterations in the resulting offspring. Franklin, TB, et al. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry 2010; 68, 408–15. In a mouse model, mice were exposed to chronic and unpredictable maternal separation from postnatal day 1 to 14. Alterations in the profile of DNA methylation in the promoter of several candidate genes in the germline of the separated males were observed. Comparable changes in DNA methylation are also present in the brain of the offspring and are associated with altered gene expression. Weiss, IC, et al. Inheritable effect of unpredictable maternal separation on behavioral responses in mice. Front Behav Neurosci 2011; 5,3. In a mouse model, unpredictable maternal separation from birth to postnatal day 14 in C57Bl/6J mice has mild behavioral effects in the animals when adult, but that its combination with maternal stress exacerbates this effect. Further, the behavioral deficits are transmitted to the following generation through females, an effect that is independent of maternal care and is not affected by cross-fostering. The combined manipulation does not alter basic components of the hypothalamic–pituitary–adrenal axis but decreases the expression of the corticotropin releasing factor receptor 2 (CRFR2) in several nuclei of the amygdala and the hypothalamus in the brain of maternal-separated females. Gapp, K. et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014. doi:10.1038/nn.3695 In a mouse model, injection of sperm RNAs from traumatized males into fertilized wild-type oocytes reproduced the behavioral and metabolic alterations in the resulting offspring. Bohacek, J. et al. Pathological brain plasticity and cognition in the offspring of males subjected to postnatal traumatic stress. Mol. Psychiatry 2014; doi:10.1038/mp.2014.80 In a mouse model, males subjected to postnatal traumatic stress have offspring with defects associated with impaired long-term memory. The expression in offspring of brain-specific signaling component in the hippocampus is reduced in the offspring, and DNA methylation at its promoter is altered both in the hippocampus of the offspring and the sperm of fathers. Razoux, F. et al. Transgenerational disruption of functional 5-HT1AR-induced connectivity in the adult mouse brain by traumatic stress in early life. Mol. Psychiatry 2016. doi:10.1038/mp.2016.146 In a mouse model, traumatic stress in postnatal life alters 5-HT1A receptor-evoked local and global functions in progeny of the exposed animals. Disrupted functional connectivity is consistent across generations and match limbic circuits implicated in mood disorders. Mechanism papers It’s also worth including a few other papers exploring mechanisms of nongenetic inheritance in mammals. Zhang Y, et al. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nat Cell Biol 2018; 20(5):535-540. Benito E, et al. RNA-Dependent Intergenerational Inheritance of Enhanced Synaptic Plasticity after Environmental Enrichment. Cell Rep 2018; 23(2):546-554. Dickson DA, et al. Reduced levels of miRNAs 449 and 34 in sperm of mice and men exposed to early life stress. Translational Psychiatry May, 2018. Sharma U, et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 31 January 2015. Huypens P, et al. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat Genetics 2016;48:497-499. Grandjean V, et al. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci Rep 2015;5:18193. So. is there really such a dearth of findings in mammal studies in epigenetic inheritance? Really? Really?! Can any reasonable person interpret the studies and conclude “no convincing evidence of intergenerational epigenetic inheritance in mammals”? How is that even possible? Wouldn’t a more reasonable response to the literature be, “Golly, such a plethora of evidence of exposure-induced intergenerational epigenetic inheritance in mammals, this sure looks like a phenomenon we should take rather seriously”? Or, to borrow a line from the 2006 paper cited by Mitchell, “Non-Mendelian inheritance, such as this, is of particular interest if it exists in humans, since it would have a profound effect on the inheritance of phenotypic traits.” [By the way, for the uber-curious, I list several reviews of these topics in Appendix 2.] Conclusion Sloppy overstatement and dogmatism from the Ivory Tower, such as Mitchell’s blogpost, can breed complacency precisely at a time when we should be deeply alarmed about the intergenerational effects of past and current exposures. It should be clear to all of us by now that molecular insults to the germline can influence disease, behavior or physiology of offspring, perhaps in ways that are staggeringly important for public health. While healthy skepticism is always welcome, research does not progress by allowing outspoken academicians to distort the state of the science, unchallenged.
Appendix 1: Overview of commonly referenced critical windows for nongenetic inheritance, by sex A. Exposures to females 1. Exposure to an adult gestating female. This exposure involves a direct exposure to a gestating F0 female, the F1 embryo/fetus and the F2 embryonic/fetal germ cells residing within. (a) The directly exposed female is the F0, implying possible impacts to her somatic cells. (b) The directly exposed male or female embryo/fetus is the F1 child soma. This is usually referred to as a “prenatal” or “fetal” exposure. Because it involves only somatic effects it is not considered part of epigenetic inheritance. (c) The male or female F1’s directly exposed embryonic/fetal germ cells (F2) later yields male or female grandchildren, or F2 genotype/epigenotype and F2 phenotype. This is usually referred to as “intergenerational” inheritance. Most of the studies cited by Mitchell involve intergenerational, and not transgenerational, inheritance. Because embryonic/fetal germ cells are highly dynamic featuring multiple levels of reprogramming (more on this later) this stage is considered an important critical window for exposure effects. (d) The F3 are the great-grandchildren of the gestating female. Effects of any F0 gestational exposure are considered “transgenerational” involving no direct exposure either to the adult F2’s germline or F3 soma. None of the studies cited by Mitchell examine this transgenerational effect, though some use the word “transgenerational” to generally mean anything past an F0 impact. 2. Exposure to a neonate female. Because the delicate process of genomic imprinting continues in the female ovary well after birth and through the first year, the infancy of the female may also present a critical window for exposures, though the molecular impacts are likely to be different than those seen in the primordial germ cell. An impact on the next generation borne of those infancy-exposed eggs would be considered intergenerational. 3. Exposure to a slow-growth period female. The period before puberty. Next generation effects would be considered “intergenerational.” 4. Exposure to an adult pre-conception, non-gestating female. Some studies consider stressors to an adult female in the period well before or shortly before conception. I don’t see a lot of evidence this window is a critical period of vulnerability for the egg epigenome, but I could be wrong. That said, a pre-conception exposure of interest need not occur just before conception to be biologically relevant. A drug or chemical can be slow to metabolize, or may lodge in the fatty tissues even for decades (think of DDT or dioxin, for example). So long-gone exposures may still directly impact the ovulated egg (intergenerational), the conceptus (somatic), or the developing embryo (somatic). Real-life biology is complicated, alas. B. Exposures to males Because males don’t gestate babies, and generate sperm instead of eggs, male-line epigenetic inheritance is a different ballgame. 1. Exposure to germ cells of a F1 embryonic/fetal male gestating in F0 female. This was discussed in A(1)(c), above. Offspring of F1 germ cells effects would be considered “intergenerational” (grandchild of the exposed gestating female). The following generation, great-grandchild (F3) of gestating female effects would be “transgenerational,” involving no direct exposure to those germ cells or soma. 2. Exposure to a neonate male. Similar to A(2), above, except that the male germ cells are apparently done with the imprinting process before birth. Next generation effects would be considered “intergenerational.” The following generation effects would be “transgenerational.” 3. Exposure to a slow-growth period male. The period before puberty. Next generation effects would be considered “intergenerational.” 4. Exposure to an adult pre-conception male. The final phase of spermatogenesis, the approximately 72 days when primary spermatocyte matures into sperm. Next generation effects would be considered “intergenerational.” The same complex considerations in A(4) above apply here. Appendix 2: Some reviews of interest Bale, TL. Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci 2015;16:332–344. Pembrey, M, et al. Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. J Med Genet 2014;51:563–572. Alonso-Magdalena, P, et al. Bisphenol-A and metabolic diseases: epigenetic, developmental and transgenerational basis. Environ Epigenetics 2016:2. Xin, F, et al. Multigenerational and transgenerational effects of endocrine disrupting chemicals: A role for altered epigenetic regulation? Semin Cell Dev Biol. 2015;43:66–75. Ozgyin, L, et al. Nuclear receptors in transgenerational epigenetic inheritance. Prog Biophys Mol Biol 2015;118:34–43. Skinner, M. Endocrine disruptor induction of epigenetic transgenerational inheritance of disease. Mol. Cell. Endocrinology 2014;398:4–12. Dunn, GA ,et al. Sex-specificity in transgenerational epigenetic programming. Horm Behav 2011;59:290–295. Gapp K, et al. . Epigenetic germline inheritance in mammals: looking to the past to understand the future. Genes, Brain and Beh 2018;17:3,e12407. Soubry A, et al. A paternal environmental legacy: evidence for epigenetic inheritance through the male germ line. Bioessays 2014;36:359–71. Soubry, A. POHaD: why we should study future fathers. Environ Epigenetics 2018; 4:2. Bohacek, J, et al. Epigenetic Inheritance of Disease and Disease Risk. Neuropsychopharmacology 2013;38:220–236. Bohacek J, et al, Transgenerational Epigenetic Effects on Brain Functions, Biol Psych 2013;73(4) 313-320. Barlow DP, et al. Genomic imprinting in mammals. CSH Perspect Biol 2014;6(2). And for those seeking an even longer read, a newly published book: Bonduriansky and Day, Extended Heredity, Princeton 2018. Jill Escher is a research philanthropist who funds pilot studies investigating exposure-induced nongenetic inheritance (Escher Fund for Autism). She is also president of Autism Society San Francisco Bay Area, a housing provider to adults with developmental disabilities, a former lawyer, and the mother of two children with idiopathic nonverbal autism. Learn more about her research philanthropy and science advocacy at GermlineExposures.org.
1 Comment
Martina Nagler
7/4/2019 07:50:44 pm
Hello, a big relief to me reading your critics on Kevin Mitchell's hypothesis. I have seen his talk at Royal Institute and thought that epigenetics was left out completely. It took me back to medieval times with DNA determinism.
Reply
Your comment will be posted after it is approved.
Leave a Reply. |
AuthorJill Escher, Escher Fund for Autism, is a California-based science philanthropist and mother of two children with severe autism, focused on the question of how environmentally induced germline disruptions may be contributing to today's epidemics of neurodevelopmental impairment. You can read about her discovery of her intensive prenatal exposure to synthetic hormone drugs here. Jill is also president of Autism Society San Francisco Bay Area. Archives
July 2021
Categories |
- Home
-
Expert Q&A
- Eva Jablonka Q&A
- Amander Clark Q&A
- Mirella Meyer-Ficca Q&A
- Janine LaSalle Q&A
- Dana Dolinoy Q&A
- Ben Laufer Q&A
- Tracy Bale Q&A
- Susan Murphy Q&A
- Alycia Halladay Q&A
- Wendy Chung Q&A
- Pradeep Bhide Q&A
- Pat Hunt Q&A
- Yauk and Marchetti Q&A
- Emilie Rissman Q&A
- Carol Kwiatkowski Q&A
- Linda Birnbaum Q&A
- Virender Rehan Q&A
- Carlos Guerrero-Bosagna Q&A
- Randy Jirtle Q&A
- Jerry Heindel Q&A
- Cheryl Walker Q&A
- Eileen McLaughlin Q&A
- Carmen Marsit Q&A
- Marisa Bartolomei Q&A
- Christopher Gregg Q&A
- Andrea Baccarelli Q&A
- David Moore Q&A
- Patrick Allard Q&A
- Catherine Dulac Q&A
- Lucas Argueso Q&A
- Toshi Shioda Q&A
- Miklos Toth Q&A
- Piroska Szabo Q&A
- Reinisch Q&A
- Klebanoff Q&A
- Denis Noble Q&A
- Germline in the News
- Science
- Presentations
- About Us
- Blog
Proudly powered by Weebly


 RSS Feed
RSS Feed